Press release
Catheter-related Bloodstream Infections Market Report 2034 | Citius Pharmaceuticals, CorMedix, Geistlich Pharma, TauroPharm GmbH, Fresenius Medical Care, expected to boost the market share
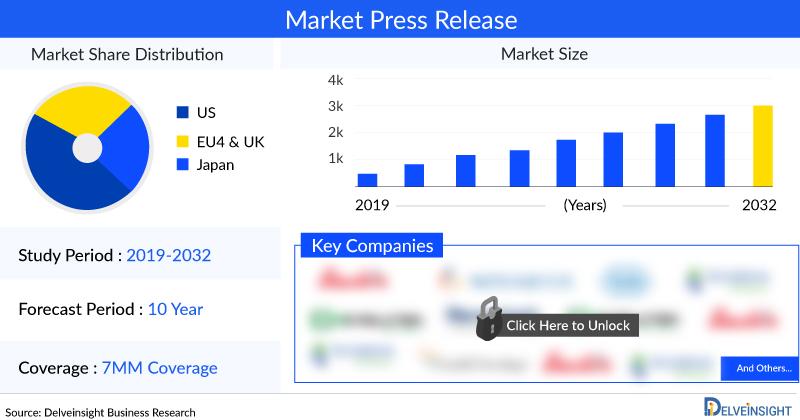
DelveInsight's "Catheter-related Bloodstream Infections Market Insights, Epidemiology, and Market Forecast-2034" report delivers an in-depth understanding of Catheter-related Bloodstream Infections, historical and forecasted epidemiology as well as the Catheter-related Bloodstream Infections market trends in the United States, EU5 (Germany, Spain, Italy, France, and United Kingdom), and Japan.The Catheter-related Bloodstream Infections market report provides current treatment practices, emerging drugs, the market share of the individual therapies, and the current and forecasted Catheter-related Bloodstream Infections market size from 2020 to 2034, segmented by seven major markets. The Report also covers current Catheter-related Bloodstream Infections treatment practice/algorithm, market drivers, market barriers, and unmet medical needs to curate the best opportunities and assesses the underlying potential of the Catheter-related Bloodstream Infections market.
Request for a Free Sample Report @ https://www.delveinsight.com/sample-request/catheter-related-bloodstream-infection-market?utm_source=openpr&utm_medium=pressrelease&utm_campaign=kpr
Key highlights from the Catheter-related Bloodstream Infections market report:
Citius Pharmaceuticals has licensed the worldwide rights to MINO-LOK from the University of Texas MD Anderson Cancer Center. MINO-LOK has received both Qualified Infectious Disease Product (QIDP) and Fast Track Designation (FTD) from the FDA, and it is protected by patents until 2024 and formulation patents until 2036.
In November 2023, CorMedix's DEFENCATH received FDA approval for treating catheter-related bloodstream infections (CRBSIs) in adult patients with kidney failure undergoing chronic hemodialysis through a central venous catheter. Gaining approval in Europe and Japan could further enhance its market potential.
In May 2024, Citius Pharmaceuticals announced positive topline results from its pivotal Phase III clinical trial of Mino-Lok, which met its primary endpoint, showing a statistically significant improvement in the time to failure events compared to the control group receiving clinician-directed anti-infective lock solutions.
The incidence of CRBSIs, the speed at which they develop, and their associated clinical burden are not well-established. The pipeline for treating catheter-related bloodstream infections is limited, with Citius Pharmaceuticals being the only company actively developing a specific drug for this condition.
An increase in the incidence of CRBSIs and the anticipated launch of new therapies are expected to drive market growth in the forecast period.
Catheter-related Bloodstream Infections Overview
Catheter-related bloodstream infections (CRBSIs) are serious complications that occur when bacteria or fungi enter the bloodstream through a central venous catheter (CVC). These infections can lead to severe health issues, including sepsis, prolonged hospitalization, and increased healthcare costs.
Causes
CRBSIs are typically caused by:
Microbial Contamination: Pathogens can enter the bloodstream from the skin, the catheter surface, or the infusion solution.
Improper Insertion Techniques: Non-sterile techniques during catheter placement can introduce microorganisms.
Prolonged Catheter Use: Longer dwell times increase the risk of infection.
Patient Factors: Immunocompromised patients, those with diabetes, or individuals undergoing chemotherapy are at higher risk.
Common pathogens associated with CRBSIs include:
Staphylococcus aureus
Coagulase-negative staphylococci
Enterobacteriaceae
Candida species
Signs and Symptoms
Symptoms of CRBSIs can vary but may include:
Fever: Often one of the first signs.
Chills: Accompanied by shaking.
Flushing or Redness: Around the catheter insertion site.
Pain or Tenderness: At the catheter site.
Rapid Heart Rate: Increased heart rate may occur.
Hypotension: Low blood pressure can indicate severe infection.
Diagnosis
Diagnosing a CRBSI typically involves:
Clinical Evaluation: Assessing symptoms and patient history.
Blood Cultures: Collecting blood samples from the catheter and a peripheral site to identify pathogens.
Catheter Tip Culture: Analyzing the catheter tip after removal to check for infection.
Imaging Studies: Ultrasound or CT scans may be used to detect complications like abscesses.
Treatment Options
The management of CRBSIs includes:
Antibiotic Therapy: Empirical treatment is started based on the most likely pathogens, guided by blood culture results.
Common antibiotics may include vancomycin, cefepime, or piperacillin-tazobactam.
Removal of the Catheter: In most cases, the infected catheter must be removed to effectively treat the infection.
Supportive Care: Monitoring vital signs, fluid resuscitation, and addressing any complications such as sepsis.
Preventive Measures: To reduce the risk of future CRBSIs, implementing strict aseptic techniques during catheter insertion and maintenance is crucial, as well as using antimicrobial-impregnated catheters when appropriate.
CRBSIs are significant complications that require prompt diagnosis and treatment. Understanding the causes, recognizing symptoms, and implementing effective treatment strategies are essential for improving patient outcomes and preventing recurrence. If you suspect a CRBSI, seek medical attention immediately.
Learn more about Catheter-related Bloodstream Infections, treatment algorithms in different geographies, and patient journeys. Contact to receive a sample @ https://www.delveinsight.com/report-store/catheter-related-bloodstream-infection-market?utm_source=openpr&utm_medium=pressrelease&utm_campaign=kpr
Catheter-related Bloodstream Infections Market
If an infection is suspected, guidelines recommend promptly removing the catheter. If the patient remains febrile after removal, antibiotics and antifungal treatments are advised, often starting with empirical therapy. Commonly prescribed off-label antibiotics for managing catheter-related bloodstream infections include daptomycin, vancomycin, cefazolin, ampicillin, ciprofloxacin, amikacin, and teicoplanin. Antifungals such as fluconazole and amphotericin B are also indicated as off-label treatments.
While the therapeutic landscape for managing catheter-related bloodstream infections is evolving, changes are occurring at a moderate pace, primarily because only one pharmaceutical company is in the late stages of developing a novel solution for this condition.
Key players like Citius Pharmaceuticals are actively evaluating their lead candidates across different stages of clinical development, aiming to explore their products for treating catheter-related bloodstream infections.
DEFENCATH, recently approved in the US for this indication, has the potential to significantly expand its market size with expected approvals in Europe and Japan. Increased patient awareness, updated diagnostic and treatment guidelines, and a growing affected population are projected to drive market growth during the forecast period. Among the seven major markets (7MM), the United States represented the largest market size for catheter-related bloodstream infections in 2023.
Request a sample and discover more about the report offerings at:
https://www.delveinsight.com/sample-request/catheter-related-bloodstream-infection-market?utm_source=openpr&utm_medium=pressrelease&utm_campaign=kpr
Catheter-related Bloodstream Infections Epidemiology
Among the seven major markets (7MM), the United States reported the highest number of incident cases in 2023. The analysis indicates that in the US, the incidence of Gram-positive bacteria exceeds that of Gram-negative bacteria, with only a small number of cases attributed to Candida species (fungi). Methicillin-susceptible Staphylococcus aureus accounts for the largest patient pool among the Gram-positive bacteria.
Within the EU4 and the UK, Germany recorded the highest number of incident cases of catheter-related bloodstream infections, while Spain reported the fewest cases in 2023.
Explore more about Catheter-related Bloodstream Infections Epidemiology at: https://www.delveinsight.com/report-store/catheter-related-bloodstream-infection-market?utm_source=openpr&utm_medium=pressrelease&utm_campaign=kpr
Catheter-related Bloodstream Infection Emerging Drugs
MINO-LOK: Citius Pharmaceuticals
MINO-LOK is an antibiotic lock solution designed to treat patients with catheter-related bloodstream infections and central line-associated bloodstream infections (CLABSIs). It is the only therapy currently under investigation for salvaging infected central venous catheters. In a Phase IIb trial, MINO-LOK achieved a 100% efficacy rate in salvaging colonized central venous catheters, with no significant adverse events reported, compared to an 18% rate of serious adverse events when infected catheters were removed and replaced. A multicenter Phase III pivotal superiority trial is now underway.
MINO-LOK consists of a proprietary blend of minocycline, edetate (disodium EDTA), and ethyl alcohol, which work synergistically to disrupt bacterial biofilms, eradicate bacteria, prevent clot formation, and maintain catheter patency. It is administered in two-hour locking cycles, allowing the central venous catheter to be used for its intended purposes for the remaining 22 hours each day.
Catheter-related Bloodstream Infections Pipeline Development Activities
The Catheter-related Bloodstream Infections report provides insights into different therapeutic candidates in Phase II, and Phase III stages. It also analyses Catheter-related Bloodstream Infections key players involved in developing targeted therapeutics.
Request for a sample report to understand more about the Catheter-related Bloodstream Infections pipeline development activities at: https://www.delveinsight.com/sample-request/catheter-related-bloodstream-infection-market?utm_source=openpr&utm_medium=pressrelease&utm_campaign=kpr
Catheter-related Bloodstream Infections Therapeutics Assessment
Major pharma companies such as Citius Pharmaceuticals, CorMedix, Geistlich Pharma, TauroPharm GmbH, Fresenius Medical Care, and others are working proactively in the Catheter-related Bloodstream Infections Therapeutics market to develop novel therapies which will drive the Catheter-related Bloodstream Infections treatment markets in the upcoming years.
Learn more about the emerging therapies & key companies at: https://www.delveinsight.com/report-store/catheter-related-bloodstream-infection-market?utm_source=openpr&utm_medium=pressrelease&utm_campaign=kpr
Catheter-related Bloodstream Infections Report Key Insights
1. Catheter-related Bloodstream Infections Patient Population
2. Catheter-related Bloodstream Infections Market Size and Trends
3. Key Cross Competition in the Catheter-related Bloodstream Infections Market
4. Catheter-related Bloodstream Infections Market Dynamics (Key Drivers and Barriers)
5. Catheter-related Bloodstream Infections Market Opportunities
6. Catheter-related Bloodstream Infections Therapeutic Approaches
7. Catheter-related Bloodstream Infections Pipeline Analysis
8. Catheter-related Bloodstream Infections Current Treatment Practices/Algorithm
9. Impact of Emerging Therapies on the Catheter-related Bloodstream Infections Market
Table of Contents
1. Key Insights
2. Executive Summary
3. Catheter-related Bloodstream Infections Competitive Intelligence Analysis
4. Catheter-related Bloodstream Infections Market Overview at a Glance
5. Catheter-related Bloodstream Infections Disease Background and Overview
6. Catheter-related Bloodstream Infections Patient Journey
7. Catheter-related Bloodstream Infections Epidemiology and Patient Population
8. Catheter-related Bloodstream Infections Treatment Algorithm, Current Treatment, and Medical Practices
9. Catheter-related Bloodstream Infections Unmet Needs
10. Key Endpoints of Catheter-related Bloodstream Infections Treatment
11. Catheter-related Bloodstream Infections Marketed Products
12. Catheter-related Bloodstream Infections Emerging Therapies
13. Catheter-related Bloodstream Infections Seven Major Market Analysis
14. Attribute Analysis
15. Catheter-related Bloodstream Infections Market Outlook (7 major markets)
16. Catheter-related Bloodstream Infections Access and Reimbursement Overview
17. KOL Views on the Catheter-related Bloodstream Infections Market
18. Catheter-related Bloodstream Infections Market Drivers
19. Catheter-related Bloodstream Infections Market Barriers
20. Appendix
21. DelveInsight Capabilities
22. Disclaimer
Get the Detailed TOC of the Catheter-related Bloodstream Infections Market report here: https://www.delveinsight.com/sample-request/catheter-related-bloodstream-infection-market?utm_source=openpr&utm_medium=pressrelease&utm_campaign=kpr
Contact Us:
Kritika Rehani
info@delveinsight.com
+14699457679
www.delveinsight.com
About DelveInsight
DelveInsight is a leading Life Science market research and business consulting company recognized for its off-the-shelf syndicated market research reports and customized solutions to firms in the healthcare sector.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Catheter-related Bloodstream Infections Market Report 2034 | Citius Pharmaceuticals, CorMedix, Geistlich Pharma, TauroPharm GmbH, Fresenius Medical Care, expected to boost the market share here
News-ID: 3694113 • Views: …
More Releases from DelveInsight Business Research LLP
Chronic Liver Disease Market Report 2032 | Major players involved- Novo Nordisk …
DelveInsight's "Chronic Liver Disease Market Insights, Epidemiology, and Market Forecast-2032" report delivers an in-depth understanding of Chronic Liver Disease, historical and forecasted epidemiology as well as the Chronic Liver Disease market trends in the United States, EU5 (Germany, Spain, Italy, France, and United Kingdom), and Japan.
The Chronic Liver Disease market report provides current treatment practices, emerging drugs, the market share of the individual therapies, and the current and forecasted…
Food Allergy Market to Rise by 2034 | Aimmune Therapeutics, Inc., Novartis Pharm …
DelveInsight's "Food Allergy Market Insights, Epidemiology, and Market Forecast-2034" report delivers an in-depth understanding of Food Allergy, historical and forecasted epidemiology as well as the Food Allergy market trends in the United States, EU5 (Germany, Spain, Italy, France, and United Kingdom), and Japan.
The Food Allergy market report provides current treatment practices, emerging drugs, the market share of the individual therapies, and the current and forecasted Food Allergy market size…

Diabetic Peripheral Neuropathy Pipeline Therapeutics, Treatment Drugs, and Compa …
DelveInsight's, "Diabetic Peripheral Neuropathy Pipeline Insight 2024" report provides comprehensive insights about 10+ companies and 15+ pipeline drugs in Diabetic Peripheral Neuropathy pipeline landscape. It covers the pipeline drug profiles, including clinical and nonclinical stage products. It also covers the therapeutics assessment by product type, stage, route of administration, and molecule type. It further highlights the inactive pipeline products in this space.
Explore our comprehensive Diabetic Peripheral Neuropathy Pipeline Report…

Amyotrophic Lateral Sclerosis Pipeline Therapeuetics Report 2024
DelveInsight's, "Amyotrophic Lateral Sclerosis Pipeline Insight 2024" report provides comprehensive insights about 75+ companies and 80+ pipeline drugs in Amyotrophic Lateral Sclerosis pipeline landscape. It covers the Amyotrophic Lateral Sclerosis pipeline drug profiles, including clinical and nonclinical stage products. It also covers the Amyotrophic Lateral Sclerosis therapeutics assessment by product type, stage, route of administration, and molecule type. It further highlights the inactive pipeline products in this space.
Explore our…
More Releases for Catheter
Exclusive Report on Catheter Stabilization Devices Catheter Securement Devices M …
According to the study published by Evolve business intelligence, “The global Catheter Stabilization Devices Catheter Securement Devices market size is expected to reach $ Billion by 2028 growing at the CAGR of 15% from 2021 to 2028. The report provides the reader with a deep-dive understanding of business opportunities, competitor analysis, market size and forecast, go-to-market strategy, and market share. After evaluating the next-gen business analytic report, the reader is…
Continuous Peripheral Nerve Block Catheter Market Report 2018: Segmentation by T …
Global Continuous Peripheral Nerve Block Catheter market research report provides company profile for Ambu, Epimed, Pajunk, B. Braun Melsungen, Teleflex, Halyard and Others.
This market study includes data about consumer perspective, comprehensive analysis, statistics, market share, company performances (Stocks), historical analysis 2012 to 2017, market forecast 2018 to 2025 in terms of volume, revenue, YOY growth rate, and CAGR for the year 2018 to 2025, etc. The report also provides…
Balloon Catheter Market Report 2018: Segmentation by Product (Normal Balloon Cat …
Global Balloon Catheter market research report provides company profile for B. Braun Melsungen, Jotech, QX Medical, Meril Life, Hexacath, Boston Scientific, Medtronic, Terumo Corporation, MicroPort Scientific Corporation and Others.
This market study includes data about consumer perspective, comprehensive analysis, statistics, market share, company performances (Stocks), historical analysis 2012 to 2017, market forecast 2018 to 2025 in terms of volume, revenue, YOY growth rate, and CAGR for the year 2018 to…
Catheter Stabilization Device/ Catheter Securement Device Market Trends and Fore …
Catheter stabilization devices are being increasingly employed in minimally invasive operations and surgeries. The catheter is stabilized or secured in order to avoid unintentional removal, decrease trauma to the bladder and the urethra, and reduce the tissues inflammation. The primary objective of catheter securement is to put a halt to the excessive traction or pull on the catheter. There are several procedures that are employed for stabilizing a catheter such…
Global Catheter Stabilization Device-Catheter Securement Devices Market Future F …
To Understand the medical equipments Industry Status worldwide, Market Research Hub (MRH) has included the recent Forecast report titled “Global Catheter Stabilization Device-Catheter Securement Devices Market Research Report 2017”, to its vast database. This study offers data about the prime regions operating in the medical equipments sector, along with their production, consumption, revenue and market share details. Further, the intelligent report also anticipates that the market would grow at a…
Catheter Stabilization Device/ Catheter Securement Device Market Research Report …
Catheter stabilization devices are being increasingly employed in minimally invasive operations and surgeries. The catheter is stabilized or secured in order to avoid unintentional removal, decrease trauma to the bladder and the urethra, and reduce the tissues inflammation. The primary objective of catheter securement is to put a halt to the excessive traction or pull on the catheter. There are several procedures that are employed for stabilizing a catheter such…