Press release
ECA Foundation releases free GMP WebApp
The ECA Foundation has been providing advanced training and information services in the pharmaceutical industry and especially with regard to pharmaceutical Quality Assurance and GMP compliance for more than 10 years. Now the organisation took advantage of its extensive experience to develop a further free of charge service – the new GMP WebApp.This new GMP WebApp runs on all smartphones and tablet PCs (Apple and Android platforms) and allows users to have GMP information at hand any time and anywhere. Due to its development as a web-based app there are no updates that need to be continuously downloaded. Any time new information is available on ECA’s website the app also displays the latest version.
The app comprises an entire set of features: users have access to all news also issued in the weekly free of charge GMP Newsletter as well as to major GMP Guidelines from authorities worldwide. Moreover, the app provides a comfortable GMP Search function. This function can be used to search simply all ECA databases, just guidelines or course and conference materials.
An additional function is an exclusive service for ECA Members: after login they have access to ECA’s Guideline Manager. Altogether this Guideline Manager includes more than 1.200 GMP Guidelines from EU/EMA, FDA, ICH, PIC/S, APIC, IPEC and WHO. In two webtrees these Guidelines are either displayed according to the issuing authorities or by GMP topics.
The ECA GMP WebApp is a free of charge service and can be opened by visiting app.gmp-compliance.org in any smartphone or tablet PC browser. To learn more please visit www.gmp-compliance.org/eca_app.html
About the ECA Foundation
Founded as an independent membership organisation in 1999, the ECA Foundation is Europe’s leading association with regard to pharmaceutical Quality Assurance and GMP compliance. Close to 4.000 members from all over Europe and abroad represent more than 60 countries.
The Foundation’s goal is “the exchange of information between representatives of the industry, regulatory authorities and universities in the field of pharmaceutical quality assurance, especially with regard to the area of Good Manufacturing Practice (GMP).” For that purpose the organisation developed a range of tools like its website for providing information on an interpretation of regulations, the weekly free of charge GMP Newsletter, the GMP Guideline Manager CD-ROM as well as its internationally acknowledged advanced education course, conference and webinar programme.
The ECA Foundation is comprised of a non-profit educational organisation (ECA Academy) and various non-profit interest groups like the European QP Association or, most recently, the Good Distribution Practices (GDP) Group.
For further information on the ECA Foundation please visit www.gmp-compliance.org.
ECA Foundation
Contact: Wolfgang Heimes
P.O. Box 10 21 68
69011 Heidelberg
Germany
www.eca-foundation.org
heimes@gmp-comliance.org
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release ECA Foundation releases free GMP WebApp here
News-ID: 273702 • Views: …
More Releases from ECA Foundation

Paul-Ehrlich-Institute and ECA Foundation jointly organise 2-day Workshop – Go …
Vaccine development and deployment are crucial to control the COVID-19 pandemic. Platform technologies have enabled rapid development of vaccines with demonstrated efficacy against COVID-19. However, large-scale manufacturing is both a prerequisite and a challenge in meeting the global demand. This implies upscaling of manufacturing, meeting Good Manufacturing Practice (GMP) requirements, and switching of manufacturing in existing facilities.
The workshop organised by the Paul-Ehrlich-Institut and the ECA Foundation will address these…

ECA Foundation added to EMA List of Stakeholder Organisations
The ECA Foundation is now listed in the European Medicines Agency's (EMA) list of eligible industry stakeholder organisations (www.ema.europa.eu/en/documents/other/list-eligible-industry-stakeholder-organisations_en.pdf). Pre-requisite for this entry is the listing in the EU's Transparency Register (http://ec.europa.eu/transparencyregister/public/consultation/searchControllerPager.do?declaration=ECA%20Foundation&search=search&locale=en#en) - where the ECA has been registered since January 2019.
The ECA Foundation is the leading European organisation with regard to pharmaceutical Quality Assurance and GMP compliance.
The ECA Foundation is a not for profit organisation. The ECA is headed…
European GDP Association nominates new Advisory Board Member
So far the Board of the European GDP Association comprised four members from the industry side, supported by a representative from the Finnish Medicines Agency FIMEA. Now a new Board Member has been nominated on the industry side.
Dr Laura Ribeiro, who is a Responsible Person at ID Logistics (formerly Logiters) in Portugal, has recently accepted her nomination as the fifth member of the GDP Association's Board. Prior to her…
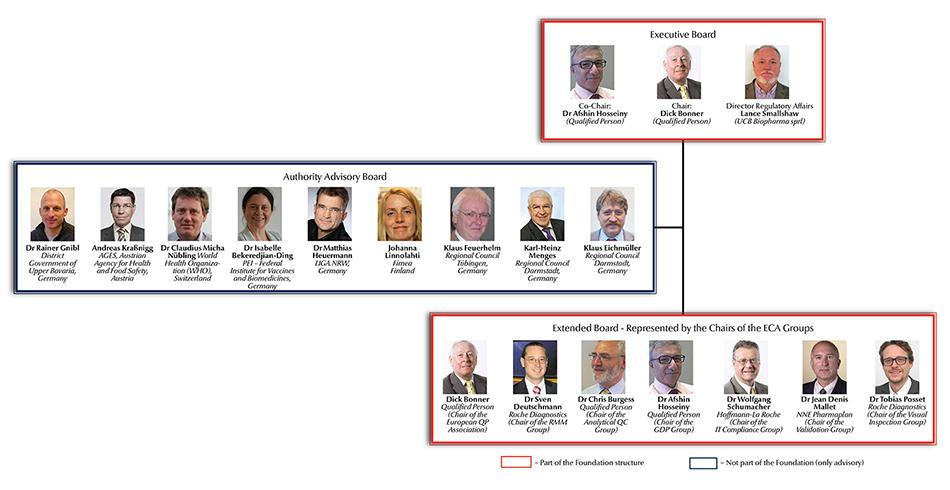
ECA Foundation announces Independent Authority Board (IAB) Members
Since June 2015 the ECA Foundation, the leading European organisation in the pharmaceutical quality assurance and GMP compliance environment, has been managed by an Executive Board and an Extended Board. In addition, the ECA set up an Advisory Committee encompassing authority representatives. Now the Foundation announced the members of the Independent Authority Board (IAB).
It is one of the ECA Foundation’s major objectives to discuss GMP developments with the stakeholders…
More Releases for GMP
Creative Peptides Released GMP Synthesis Service
Located in Shirley, New York, the world’s leading peptide supplier Creative Peptides announced the launch of its GMP synthesis (https://www.creative-peptides.com/services/custom-gmp-peptide-synthesis-services.html ) business on August 29, 2018. Now this company is focused on the development and GMP manufacturing of pharmaceutical grade peptides.
As the demand of pharmaceutical market continues to grow, more and more pharmas and research institutions choose the CMO and CRO models to expand their businesses, which is more…
Diapharm implements European GMP guidelines in China
Münster (DE), London (UK), Ningbo (CN), 20 December 2013 – Pharmaceutical service provider Diapharm (diapharm.com) is increasing its business activities in China: Diapharm has now implemented a “European” quality management system for Neptune Pharma Ltd (www.neptunepharma.com) in their Joint Venture Partner’s factory in Ningbo, Zhejiang Province. And it has done so successfully: The veterinary medicinal product Trident 500mg/g Powder for Suspension for Fish Treatment (www.trident-50.com), is manufactured onsite under EU…
GMP Friction Products Awarded ISO 9001:2008
Internationally Recognized Certification Measures Consistency in Process, Procedure and Quality Performance in Manufacture of Friction Materials
AKRON, OH (March 23, 2011) -- GMP Friction Products, a world leader manufacturing powdered metal friction products for clutch plates and brake pads, recently received certification for ISO 9001:2008.
“ISO 9001:2008 signifies we have taken the extra measure of documenting the policies and standards to ensure consistent compliance with our manufacturing processes,” said Jerry Lynch,…
GMP MANUAL Volume 2 - Validation Procedures by Maas & Peither AG – GMP Publish …
GMP Publishing is launching its new GMP MANUAL Volume 2 – Validation Procedures.
The compendium on validation procedures was written by Dr. Doris Borchert, Dr. Peter Bosshard, Dr. Ralph Gomez, Dr. Michael Hiob, Dr. Christine Oechslein, Max Lazar, Ulrike Reuter, Michael Schulte, Uwe Schwarzat – all international experts and key opinion leaders. They share their detailed understanding of the various aspects of the validation process in clear and comprehensive style…
blue inspection body celebrates 50 GMP audits
Münster (Germany), 20 November 2009. Two years after founding the company and just 18 months after gaining the accreditation blue inspection body GmbH announced today the successful execution of its 50th GMP audit. Further audit trips to China, India, Israel and various European countries have been scheduled already, meaning that in the first quarter 2010 the 75th audit is targeted to be completed. Blue, as a privately organised inspection body,…
Easternpak Retains GMP & BRC Certifications
(Ajaltoun, Lebanon) 13 July 2009 – Easternpak Ltd. has successfully completed repeat audits for International Good Manufacturing Practice GMP for Corrugated & Solid Board and British Retail Consortium (BRC / IOP) certifications.
Easternpak originally received the certifications during 2007. The corrugated packaging plant in Dammam, Saudi Arabia is also Hazard Analysis & Critical Control Point (HACCP) certified and ISO 9001:2000 accredited by CERT/TUV in Germany.
Easternpak General Manager, Pierre Akl, shares,
“We continuously…
