Press release
Dry Eye Disease Pipeline Therapeutics, Assessment, Companies, Products, Unmet Needs, Market Drivers and Barriers
DelveInsight's, "Dry Eye Disease Pipeline Insight 2024" report provides comprehensive insights about 45+ companies and 50+ pipeline drugs in Dry Eye Disease pipeline landscape. It covers the pipeline drug profiles, including clinical and nonclinical stage products. It also covers the therapeutics assessment by product type, stage, route of administration, and molecule type. It further highlights the inactive pipeline products in this space.Discover the latest drugs and treatment options in the Dry Eye Disease Pipeline. Dive into DelveInsight's comprehensive report today! @ Dry Eye Disease Pipeline Outlook [https://www.delveinsight.com/sample-request/dry-eye-disease-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=ypr]
Key Takeaways from the Dry Eye Disease Pipeline Report
* In October 2024:- Stealth BioTherapeutics Inc.- The goal of this clinical trial is to evaluate the efficacy,safety and pharmacokinetics of elamipretide in subjects with dry age-related macular degeneration (AMD). The main questions it aims to answer are: what is the rate of change in the macular area of photoreceptor loss in subjects who receive a daily dose of elamipretide compared with those who receive a look-alike substance that contains no active drug, and what is the safety and tolerability of elamipretide daily subcutaneous injections.
* In October 2024:- Novartis Pharmaceuticals- The purpose of this study is to demonstrate the comparability of ianalumab exposure following the sub-cutaneous (s.c.) administration of one injection of 300 mg/2 mL auto-injector (AI) versus two injections of 150 mg/1 mL pre-filled syringe (PFS), and to evaluate the safety and tolerability of ianalumab following the s.c. administration of both devices in participants with rheumatoid arthritis (RA), Sjogren's disease (SjD), or systemic lupus erythematosus (SLE).
* In October 2024:- Hoffmann-La Roche- The main objective of the study is evaluation of the safety and tolerability of OpRegen - Human embryonic stem cell-derived retinal pigment epithelial (RPE) cells. The study will also include initial exploration of the ability of transplanted OpRegen cells to engraft, survive, and moderate disease progression.
* In October 2024:- Amgen- The study will enroll 2 SS populations: Population 1 will include participants with moderate to high systemic disease activity; Population 2 will include participants with moderate to severe subjective symptoms. This study will include 3 periods: screening (5 weeks), treatment period (48 Weeks) and follow-up period (12 weeks). In the treatment period, participants from each of the populations will be randomized to receive subcutaneous (SC) dose of HZN-1116 or placebo.
* DelveInsight's Dry Eye Disease pipeline report depicts a robust space with 45+ active players working to develop 50+ pipeline therapies for Dry Eye Disease treatment.
* The leading Dry Eye Disease Companies such as Alcon, Seikagaku Corporation, Huons, Aramis Biosciences, Invirsa, Inc., IVIEW Therapeutics, Seinda Pharmaceutical, Serentrix, EyeD Pharma, SELAGINE, Theratome Bio, Alchemedicine , and others.
* Promising Dry Eye Disease Therapies such as TJO-083, Licaminlimab, TL-925, Reproxalap Ophthalmic Solution (0.25%), AZR-MD-001, Cyclosporine ophthalmic solution, 0.1%, VSJ-110, and others.
Stay ahead with the most recent pipeline outlook for Dry Eye Disease. Get insights into clinical trials, emerging therapies, and leading companies with DelveInsight @ Dry Eye Disease Treatment Drugs [https://www.delveinsight.com/sample-request/dry-eye-disease-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=ypr]
Dry Eye Disease Emerging Drugs Profile
* AR-15512: Alcon
AR-15512 is an investigational eye drop currently in clinical development at Aerie as a potential treatment for the signs and symptoms of dry eye disease. The active ingredient in AR-15512 is a proprietary small-molecule selective agonist of the transient receptor potential melastatin 8 (TRPM8) cold thermoreceptor, which represents a novel therapeutic target for dry eye. The drug is currently in Phase III stage of clinical trial evaluation for the treatment of DED.
* SI-614: Seikagaku Corporation
SI-614, an ophthalmic solution being developed by Seikagaku Corporation. CT-868 is a. SI-614 is an amphiphilic polymer produced by introducing a hydrophobic group into hyaluronic acid using Seikagaku's own proprietary technology. Ocular instillation of SI-614 in dry eye patients is thought to stabilize the tear film by utilizing the mucoadhesive and surface tension reducing properties of SI-614 and to promote corneal epithelial wound healing by binding SI-614 to the fibronectin that occurs on corneal epithelial defects to promote epithelial cell growth. Through these actions, SI-614 is expected to restore the tear film and corneal structure to their normal state and improve symptoms associated with dry eye. Currently, the drug is in the Phase III stage of development to treat Dry Eye Disease.
* HU007: Huons
HU007 is a combination of cyclosporine, an anti-inflammatory component, and trehalose, a tear film protection component, to minimize eye surface irritation such as burning sensation by lowering the concentration of cyclosporine to less than half than that of existing treatments. In addition, it is an ophthalmic agent developed to suppress the destruction of each conjunctival epithelial cells caused by drying by combining trehalose preparations and to see the combined treatment effect for dry eyes. Currently, the drug is in the Phase III stage of development to treat Dry Eye Disease.
* A197: Aramis Biosciences
A197 is a novel topical agent that targets the immunopathogenesis of dry eye disease. It has a unique mechanism of action compared to existing dry eye treatments. Currently, the drug is in the Phase II stage of development to treat Dry Eye Disease.
* INV 102: Invirsa, Inc.
INV 102 is an ophthalmic eye drop being developed by Invirsa, Inc. Invirsa's lead candidate is a naturally-occurring, small molecule that modulates the activity of p53, the central protein responsible for regulating the DNA damage response. Currently, the drug is in the Phase II stage of development to treat Dry Eye Disease.
* iVIEW 1001: IVIEW Therapeutics
iVIEW 1001 is a TRPM8 agonist drug being developed by iVIEW Therapeutics, Inc. to treat dry eye diseases. IVIEW 1001 is a TRPM8 agonist, which means it stimulates the transient receptor potential melastatin 8 (TRPM8) receptors in the eyelid margin. This stimulation sends a perception of coolness, reducing ocular discomfort and potentially increasing tear secretion. Currently, the drug is in the Phase I/II stage of development to treat Dry Eye Disease.
Explore groundbreaking therapies and clinical trials in the Dry Eye Disease Pipeline. Access DelveInsight's detailed report now! @ New Dry Eye Disease Drugs [https://www.delveinsight.com/sample-request/dry-eye-disease-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=ypr]
Dry Eye Disease pipeline report provides the therapeutic assessment of the pipeline drugs by the Route of Administration
* Oral
* Intravenous
* Subcutaneous
* Parenteral
* Topical
Dry Eye Disease Products have been categorized under various Molecule types such as
* Recombinant fusion proteins
* Small molecule
* Monoclonal antibody
* Peptide
* Polymer
* Gene therapy
Unveil the future of Dry Eye Disease Treatment. Learn about new drugs, pipeline developments, and key companies with DelveInsight's expert analysis @ Dry Eye Disease Market Drivers and Barriers [https://www.delveinsight.com/sample-request/dry-eye-disease-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=ypr]
Scope of the Dry Eye Disease Pipeline Report
* Coverage- Global
* Dry Eye Disease Companies- Alcon, Seikagaku Corporation, Huons, Aramis Biosciences, Invirsa, Inc., IVIEW Therapeutics, Seinda Pharmaceutical, Serentrix, EyeD Pharma, SELAGINE, Theratome Bio, Alchemedicine, and others.
* Dry Eye Disease Therapies- TJO-083, Licaminlimab, TL-925, Reproxalap Ophthalmic Solution (0.25%), AZR-MD-001, Cyclosporine ophthalmic solution, 0.1%, VSJ-110, and others.
* Dry Eye Disease Therapeutic Assessment by Product Type: Mono, Combination, Mono/Combination
* Dry Eye Disease Therapeutic Assessment by Clinical Stages: Discovery, Pre-clinical, Phase I, Phase II, Phase III
Get the latest on Dry Eye Disease Therapies and clinical trials. Download DelveInsight's in-depth pipeline report today! @ Dry Eye Disease Companies, Key Products and Unmet Needs [https://www.delveinsight.com/sample-request/dry-eye-disease-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=ypr]
Table of Content
* Introduction
* Executive Summary
* Dry Eye Disease: Overview
* Pipeline Therapeutics
* Therapeutic Assessment
* Dry Eye Disease- DelveInsight's Analytical Perspective
* Late Stage Products (Phase III)
* AR-15512: Alcon
* Drug profiles in the detailed report.....
* Mid Stage Products (Phase II)
* INV 102: Invirsa, Inc.
* Drug profiles in the detailed report.....
* Early Stage Products (Phase I/II)
* iVIEW 1001: IVIEW Therapeutics
* Drug profiles in the detailed report.....
* Preclinical and Discovery Stage Products
* Drug name: Company name
* Drug profiles in the detailed report.....
* Inactive Products
* Dry Eye Disease Key Companies
* Dry Eye Disease Key Products
* Dry Eye Disease- Unmet Needs
* Dry Eye Disease- Market Drivers and Barriers
* Dry Eye Disease- Future Perspectives and Conclusion
* Dry Eye Disease Analyst Views
* Dry Eye Disease Key Companies
* Appendix
About Us
DelveInsight is a leading healthcare-focused market research and consulting firm that provides clients with high-quality market intelligence and analysis to support informed business decisions. With a team of experienced industry experts and a deep understanding of the life sciences and healthcare sectors, we offer customized research solutions and insights to clients across the globe. Connect with us to get high-quality, accurate, and real-time intelligence to stay ahead of the growth curve.
Media Contact
Company Name: DelveInsight Business Research LLP
Contact Person: Yash Bhardwaj
Email:Send Email [https://www.abnewswire.com/email_contact_us.php?pr=dry-eye-disease-pipeline-therapeutics-assessment-companies-products-unmet-needs-market-drivers-and-barriers]
Phone: 09650213330
Address:304 S. Jones Blvd #2432
City: Las Vegas
State: NV
Country: United States
Website: https://www.delveinsight.com/
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Dry Eye Disease Pipeline Therapeutics, Assessment, Companies, Products, Unmet Needs, Market Drivers and Barriers here
News-ID: 3694573 • Views: …
More Releases from ABNewswire

Urea Prices Report 2024, Chart, News, Index, Demand and Forecast
in the U.S. urea prices experienced a decline, primarily due to a blend of moderate demand and logistical issues.
Urea Prices Analysis:
United State: 470 USD/MT
China: 350 USD/MT
Europe: 410 USD/MT
[https://www.imarcgroup.com/urea-pricing-report]. In the fourth quarter of the last year, U.S. urea prices experienced a decline, primarily due to a blend of moderate demand and logistical issues.
The latest IMARC Group report, "Urea Pricing Report 2024: Price Trend, Chart, Market Analysis, News, Demand, Historical and…
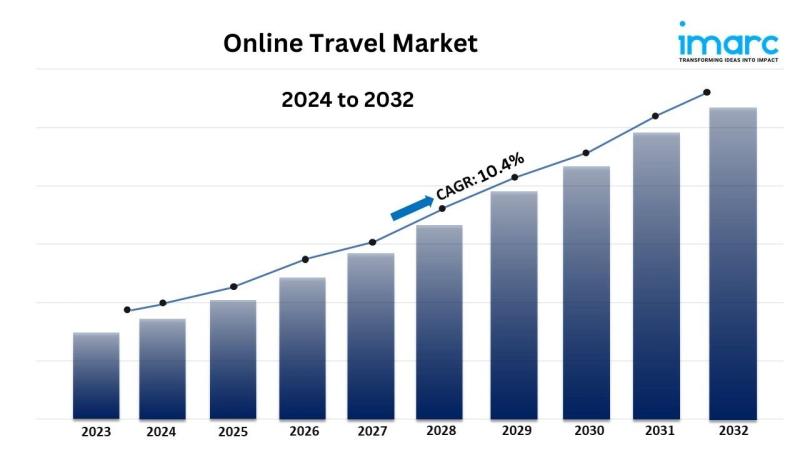
Online Travel Market Size Projected to Reach USD 512.5 Billion by 2032, Growing …
Online Travel Industry
Summary:
* The global [https://www.imarcgroup.com/online-travel-market] size reached USD 512.5 Billion in 2023.
* The market is expected to reach USD 1,267.1 Billion by 2032, exhibiting a growth rate (CAGR) of 10.4% during 2024-2032.
* North America leads the market, accounting for the largest online travel market share.
* Travel accommodation accounts for the majority of the market share in the service type segment due to the increasing…
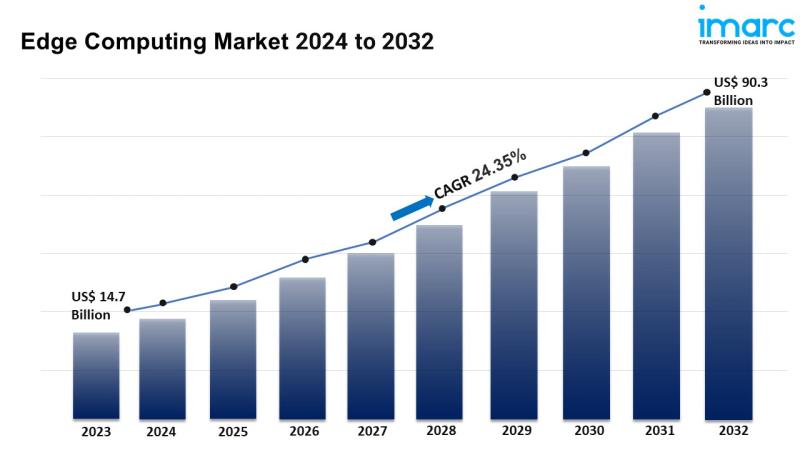
Edge Computing Market Size to Hit USD 90.3 Billion in 2032 | With a 24.35% CAGR
The global edge computing market size reached US$ 14.7 Billion in 2023. Looking forward, IMARC Group expects the market to reach US$ 90.3 Billion by 2032, exhibiting a growth rate (CAGR) of 24.35% during 2024-2032.
Summary:
* The global edge computing market size reached USD 14.7 Billion [https://www.imarcgroup.com/edge-computing-market] in2023.
* The market is expected to reach USD 90.3 Billion by 2032, exhibiting a growth rate (CAGR) of 24.35% during 2024-2032.
*…
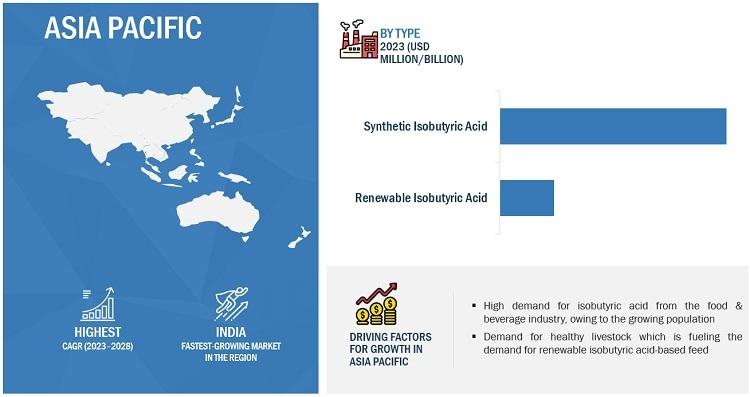
Isobutyric Acid Market Size, Trends, Market Drivers, Restraints, Opportunities, …
The Isobutyric Acid industry is seeing a steady rise in demand, driven by its applications in food, pharmaceuticals, and chemical production. With its utility in flavors, fragrances, and as an intermediate in various compounds, the market is projected to grow. Innovations in production methods and the rising focus on sustainable solutions are set to further enhance its demand across diverse sectors.
The isobutyric acid market [https://www.marketsandmarkets.com/Market-Reports/isobutyric-acid-market-262525625.html?utm_source=abnewswire.com&utm_medium=referral&utm_campaign=isobutyricacid] is projected to grow from…
More Releases for Eye
Global New Born Eye Imaging Systems Market SWOT Analysis, Key Indicators, Foreca …
New Born Eye Imaging Systems
The business report released by Zion Market Research on New Born Eye Imaging Systems Market: Global Industry Analysis, Size, Share, Growth, Trends, and Forecasts 2016–2024 market is focused to facilitate a deep understanding of the market definition, potential, and scope. The report is curated after deep research and analysis by experts. It consists of an organized and methodical explanation of current market trends to assist the…
Global New Born Eye Imaging Systems Market 2020 Business Strategies – Eye Phot …
The Zion Market Research added a new report “New Born Eye Imaging Systems Market: Global Industry Analysis, Size, Share, Growth, Trends, and Forecasts 2016–2024” in its database, which provides an expert and in-depth analysis of key business trends and future market development prospects, key drivers and restraints, profiles of major market players, segmentation and forecasting.
New Born Eye Imaging Systems Market Market research report which provides an in-depth examination of the…
Two St. Louis Area Eye Doctors Relaunch Complete Eye Safety
Two St. Louis area eye doctors, Dr. Mark Kahrhoff, OD, and Dr. Derek Wiles, OD, are relaunching Complete Eye Safety, a company providing occupational and industrial prescription safety eyewear and services to companies throughout the Midwest.
The relaunch includes a compete offering of the latest prescription safety glasses as well as "industry specific" eyewear, a new website for online ordering, proprietary services such as on-site vision tests, Optician-on-Demand, on-site eye…
Dry Eye Syndrome Treatment Market 2027 by Drugs Enhancements - Lubricant Eye Dro …
Dry Eye Syndrome or keratoconjunctivitis sicca is referred to inappropriate formation of tears that evaporates too quickly or dearth of tears that creates problem in lubrication and nourishment of the eye. This condition can lead to several disorders such as cornea, ulcers and loss of vision.
Dry Eye Syndrome Treatment Market to 2027 - Global Analysis and Forecasts by : Drugs (Lubricant Eye Drops, Anti-inflammatory Drugs, Autologous Serum Eye Drops)…
Artificial Eye Market 2019 | Global Forecast 2025 | Top Key Players - National A …
Artificial Eye Market research report delivers a close watch on leading competitors with strategic analysis, micro and macro market trend and scenarios, pricing analysis and a holistic overview of the market situations in the forecast period.
Get Exclusive FREE Sample Copy Of this Report @ https://www.upmarketresearch.com/home/requested_sample/85905
UpMarketResearch offers a latest published report on “Global Artificial Eye Market Analysis and Forecast 2019 - 2025” delivering key insights and providing a competitive advantage to…
Ophthalmic Eye Shield Market Segmentation By Material type Aluminium Ophthalmic …
Eye shields are advised for use in the eye while a periorbital surgery to avoid any injury to the globe. Ophthalmic eye shields are used for postoperative ocular protection. Ophthalmic eye shields protect the cornea and retina while any eye surgery or laser treatment. Ophthalmic eye shields are highly preferred for cataract surgeries in the elder population and blepharitis correction especially for children. According to WHO, The World Health Organization,…
