Press release
Duchenne Muscular Dystrophy Treatment Drugs, Clinical Trials, Pipeline Insights and Companies 2024
DelveInsight's, "Duchenne Muscular Dystrophy Pipeline Insight 2024" report provides comprehensive insights about 75+ companies and 75+ pipeline drugs in Duchenne Muscular Dystrophy pipeline landscape. It covers the Duchenne Muscular Dystrophy pipeline drug profiles, including clinical and nonclinical stage products. It also covers the Duchenne Muscular Dystrophy therapeutics assessment by product type, stage, route of administration, and molecule type. It further highlights the inactive pipeline products in this space.Explore our latest breakthroughs in Duchenne Muscular Dystrophy Research. Learn more about our innovative pipeline today! @ Duchenne Muscular Dystrophy Pipeline Outlook [https://www.delveinsight.com/sample-request/duchenne-muscular-dystrophy-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=ypr]
Key Takeaways from the Duchenne Muscular Dystrophy Pipeline Report
* In September 2024:- Edgewise Therapeutics Inc.- A study of sevasemten (EDG-5506) in Becker muscular dystrophy (known as CANYON) and pivotal cohort (known as GRAND CANYON). The EDG-5506-201 CANYON study was expanded to include an additional 120 adult participants in a cohort called GRAND CANYON, that is a multicenter, randomized, double-blind, placebo-controlled study to evaluate the safety and efficacy of sevasemten in adults with Becker.
* In September 2024:- Solid Biosciences Inc.- This is a multicenter, open-label, non-randomized study to investigate the safety, tolerability, and efficacy of a single intravenous (IV) infusion of SGT-003 in participants with Duchenne muscular dystrophy. There will be 2 cohorts in this study. Cohort 1 will include participants 4 to
* DelveInsight's Duchenne Muscular Dystrophy pipeline report depicts a robust space with 75+ active players working to develop 75+ pipeline therapies for Duchenne Muscular Dystrophy treatment.
* The leading Duchenne Muscular Dystrophy Companies such as Santhera Pharmaceuticals, Sarepta Therapeutics, Italfarmaco, Wave Life Sciences Ltd, FibroGen, EDG 5506 Edgewise Therapeutics, Fordadistrogene movaparvovec, Daiichi Sankyo, Sarepta Therapeutics, Inc., ENCell, Taiho Pharmaceutical, Solid Biosciences, Capricor, Nippon Shinyaku, Hansa Biopharma, and others.
* Promising Duchenne Muscular Dystrophy Therapies such as Vamorolone, Sevasemten 10 mg, Givinostat, DS-5141b, SGT-003, PF-06939926, NS-089/NCNP-02, and others.
Stay informed about the cutting-edge advancements in Duchenne Muscular Dystrophy Treatments. Download for updates and be a part of the revolution in Musculoskeletal Care @ Duchenne Muscular Dystrophy Clinical Trials Assessment [https://www.delveinsight.com/sample-request/duchenne-muscular-dystrophy-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=ypr]
Duchenne Muscular Dystrophy Emerging Drugs
* Vamorolone: Santhera
Vamorolone is a first-in-class drug candidate that binds to the same receptors as corticosteroids but modifies the downstream activity of the receptors1,2. This has the potential to 'dissociate' efficacy from typical steroid safety concerns and therefore could emerge as a valuable alternative to corticosteroids, the current standard of care in children and adolescent patients with DMD. There is a clear unmet medical need in this patient group as high dose corticosteroids have significant systemic side effects that detract from patient quality of life. On September 2, 2020, Santhera exercised its option and obtained worldwide rights to vamorolone in Duchenne muscular dystrophy and all other indications. Santhera and ReveraGen expect to complete the rolling NDA submission to the U.S. FDA in June 2022.
* Givinostat: Italfarmaco
Givinostat, is an HDAC inhibitor (HDACi, a principle candidate, currently being developed for the treatment of DMD and BMD. Since Givinostat acts on the pathogenetic events downstream of the genetic defects, it is potentially a treatment for the whole DMD and BMD population and to counter the disease pathogenetic events in all muscular districts.
* Pamrevlumab: Fibrogen
Pamrevlumab is a first-in-class antibody developed by FibroGen to inhibit the activity of connective tissue growth factor (CTGF), a common factor in fibrotic and proliferative disorders characterized by persistent and excessive scarring that can lead to organ dysfunction and failure. Pamrevlumab is advancing towards Phase 3 clinical development for the treatment of idiopathic pulmonary fibrosis (IPF) and pancreatic cancer and has been granted Orphan Drug Designation (ODD) in each of these indications, and is currently in a Phase 2 trial for Duchenne muscular dystrophy (DMD).
Learn more about Duchenne Muscular Dystrophy Drugs opportunities in our groundbreaking Duchenne Muscular Dystrophy Research and development projects @ Duchenne Muscular Dystrophy Unmet Needs [https://www.delveinsight.com/sample-request/duchenne-muscular-dystrophy-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=ypr]
Duchenne Muscular Dystrophy pipeline report provides the therapeutic assessment of the pipeline drugs by the Route of Administration
* Oral
* Intravenous
* Subcutaneous
Duchenne Muscular Dystrophy Products have been categorized under various Molecule types such as
* Small molecule
* Cell Therapy
* Peptides
* Polymer
* Small molecule
* Gene therapy
Discover the latest advancements in Duchenne Muscular Dystrophy Treatment by visiting our website. Stay informed about how we're transforming the future of musculoskeletal @ Duchenne Muscular Dystrophy Market Drivers and Barriers, and Future Perspectives [https://www.delveinsight.com/sample-request/duchenne-muscular-dystrophy-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=ypr]
Scope of the Duchenne Muscular Dystrophy Pipeline Report
* Coverage- Global
* Duchenne Muscular Dystrophy Companies- Santhera Pharmaceuticals, Sarepta Therapeutics, Italfarmaco, Wave Life Sciences Ltd, FibroGen, EDG 5506 Edgewise Therapeutics, Fordadistrogene movaparvovec, Daiichi Sankyo, Sarepta Therapeutics, Inc., ENCell, Taiho Pharmaceutical, Solid Biosciences, Capricor, Nippon Shinyaku, Hansa Biopharma, and others.
* Duchenne Muscular Dystrophy Therapies- Vamorolone, Sevasemten 10 mg, Givinostat, DS-5141b, SGT-003, PF-06939926, NS-089/NCNP-02, and others.
* Duchenne Muscular Dystrophy Therapeutic Assessment by Product Type: Mono, Combination, Mono/Combination
* Duchenne Muscular Dystrophy Therapeutic Assessment by Clinical Stages: Discovery, Pre-clinical, Phase I, Phase II, Phase III
For a detailed overview of our latest research findings and future plans, read the full details of Duchenne Muscular Dystrophy Pipeline on our website @ Duchenne Muscular Dystrophy Drugs and Companies [https://www.delveinsight.com/sample-request/duchenne-muscular-dystrophy-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=ypr]
Table of Content
* Introduction
* Executive Summary
* Duchenne Muscular Dystrophy: Overview
* Pipeline Therapeutics
* Therapeutic Assessment
* Duchenne Muscular Dystrophy- DelveInsight's Analytical Perspective
* Late Stage Products (Phase III)
* Delandistrogene moxeparvovec: Roche
* Drug profiles in the detailed report.....
* Mid Stage Products (Phase II)
* SRP 5051: Sarepta Therapeutics
* Drug profiles in the detailed report.....
* Early Stage Products (Phase I/II)
* WVE N531: Wave Life Sciences
* Drug profiles in the detailed report.....
* Early Stage Products (Phase I)
* EDG 5506: Edgewise Therapeutics
* Drug profiles in the detailed report.....
* Inactive Products
* Duchenne Muscular Dystrophy Key Companies
* Duchenne Muscular Dystrophy Key Products
* Duchenne Muscular Dystrophy- Unmet Needs
* Duchenne Muscular Dystrophy- Market Drivers and Barriers
* Duchenne Muscular Dystrophy- Future Perspectives and Conclusion
* Duchenne Muscular Dystrophy Analyst Views
* Duchenne Muscular Dystrophy Key Companies
* Appendix
About Us
DelveInsight is a leading healthcare-focused market research and consulting firm that provides clients with high-quality market intelligence and analysis to support informed business decisions. With a team of experienced industry experts and a deep understanding of the life sciences and healthcare sectors, we offer customized research solutions and insights to clients across the globe. Connect with us to get high-quality, accurate, and real-time intelligence to stay ahead of the growth curve.
Media Contact
Company Name: DelveInsight Business Research LLP
Contact Person: Yash Bhardwaj
Email:Send Email [https://www.abnewswire.com/email_contact_us.php?pr=duchenne-muscular-dystrophy-treatment-drugs-clinical-trials-pipeline-insights-and-companies-2024]
Phone: 9650213330
Address:304 S. Jones Blvd #2432
City: Las Vegas
State: NV
Country: United States
Website: https://www.delveinsight.com/
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Duchenne Muscular Dystrophy Treatment Drugs, Clinical Trials, Pipeline Insights and Companies 2024 here
News-ID: 3668131 • Views: …
More Releases from ABNewswire

Digital PCR and Real-Time PCR Market Dynamics: Forecasted Growth to USD 14.8 Bil …
The global digital PCR (dPCR) and real-time PCR (qPCR) market is set to expand significantly, with projections indicating growth from USD 10.0 billion in 2024 to USD 14.8 billion by 2029 at a CAGR of 8.1% during the forecast period of 2024 to 2029
The global digital PCR (dPCR) and real-time PCR (qPCR) market [https://www.marketsandmarkets.com/Market-Reports/digital-pcr-market-174151204.html?utm_source=abnewswire.com&utm_medium=digitalpcrmarket] is set to expand significantly, with projections indicating growth from USD 10.0 billion in 2024 to…
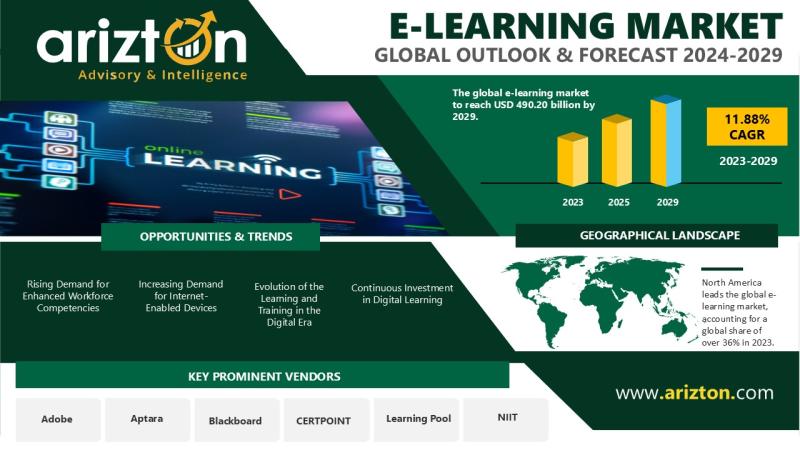
E-Learning Market to Worth $490 Billion by 2029, 2X Growth in the Next 6 Years - …
According to Arizton's latest research report, the e-learning market [https://www.arizton.com/market-reports/e-learning-market-size-2025] is growing at a CAGR of 11.88% during 2023-2029.
Looking for More Information? Click: [https://www.arizton.com/market-reports/e-learning-market-size-2025]
Report Scope:
Market Size (2029): USD 490.2 Billion
Market Size (2023): USD 250 Million
CAGR (2023-2029): 11.88%
Historic Year: 2020-2023
Base Year: 2023
Forecast Year: 2024-2029
Market Segmentation: Learning Mode, End-users, Function Type, Delivery Mode, Content Type, Technology Type, and Geography
Geographic Analysis: North America, Europe, APAC, Latin America, and Middle East & Africa
Book the…

Ascites Treatment Market Size in the 7MM is expected to grow by 2034, estimates …
Ascites Treatment Market is projected to witness substantial growth over the next few years, driven by advancements in treatment modalities and increasing awareness of the condition. The pipeline for Ascites Therapeutics includes promising candidates that aim to not only treat acute attacks but also prevent recurrence.
DelveInsight's "Ascites Market Insights, Epidemiology and Market Forecast - 2034" report delivers an in-depth understanding of the Ascites, historical and forecasted epidemiology as well…

Biomarkers Industry worth $93.8 billion in 2029, with a CAGR of 10.2%
Biomarkers Market in terms of revenue was estimated to be worth $57.7 billion in 2024 and is poised to reach $93.8 billion by 2029, growing at a CAGR of 10.2% from 2024 to 2029 according to a new report by MarketsandMarkets Trademark .
Biomarkers Market [https://www.marketsandmarkets.com/Market-Reports/biomarkers-advanced-technologies-and-global-market-43.html?utm_source=abnewswire.com&utm_medium=biomarkersmarket] in terms of revenue was estimated to be worth $57.7 billion in 2024 and is poised to reach $93.8 billion by 2029, growing at a…
More Releases for Duchenne
Duchenne Muscular Dystrophy (DMD) Drugs Market Report
As per the research conducted by GME, the Duchenne Muscular Dystrophy (DMD) Drugs Market will grow with a CAGR value of 41.3 percent by 2026. The global market for Duchenne muscular dystrophy is foreseen to grow due to accelerated research and development activities, expanded patient awareness for effective treatments, and the launch of new medications Moreover, the disease's increasing prominence is likely to drive the global duchenne muscular dystrophy market…
Global Duchenne Muscular Dystrophy Market Research Report 2015-2025
About the Global Duchenne Muscular Dystrophy Market Research Report 2015-2025:
Global Global Duchenne Muscular Dystrophy Market Research Report 2015-2025 2020 Research Report is divided into various key segments like device type, material, applications, and end users. The report is thoroughly analyzed and studied by the researchers to offer deep insights on each of these segments with statistics. This information is beneficial for the manufacturers in the industry to plan their policies…
Global Duchenne Muscular Dystrophy Therapeutics Market Productions, Statistics 2 …
Qyresearchreports include new market research report "Global Duchenne Muscular Dystrophy Therapeutics Market Size, Status and Forecast 2022" to its huge collection of research reports.
The general market for Duchenne Muscular Dystrophy Therapeutics has been analysed on various points of view that are practically present in the scenario, and have affected the market situation to the large extent. The report also presents exceptional experience and data identified with global Duchenne Muscular Dystrophy…
Duchenne Muscular Dystrophy - Pipeline Review, H2 2017
ReportsWorldwide has announced the addition of a new report title Duchenne Muscular Dystrophy - Pipeline Review, H2 2017 to its growing collection of premium market research reports.
Summary
Global Markets Direct's latest Pharmaceutical and Healthcare disease pipeline guide Duchenne Muscular Dystrophy - Pipeline Review, H2 2017, provides an overview of the Duchenne Muscular Dystrophy (Genetic Disorders) pipeline landscape.
Duchenne muscular dystrophy is a condition which causes muscle weakness. DMD is an X-linked disorder.…
Duchenne Muscular Dystrophy (DMD) Treatment Market - Global Industry Insights, T …
Duchenne muscular dystrophy (DMD) is a genetic disorder characterized by progressive muscle degeneration and weakness. DMD is caused due to alteration in gene sequence coding for dystrophin proterin, which is present the muscle. It is a rare muscle disease which majorly affects males. The symptoms includes intellectual disability, muscle weakness and, difficulty in walking and breathing. Congestive heart failure, mental impairment, pneumonia or respiratory failure are some of the…
Duchenne Muscular Dystrophy (DMD) Market Forecast Research Reports Offers Key In …
Duchenne muscular dystrophy (DMD) is a genetic disorder characterized by muscle degeneration and weakness. Duchenne muscular dystrophy (DMD) cause due to lack of protein known as “dystrophin” which causes muscles deterioration and break down, leads to difficulty in walking and general mobility. DMD is a one of the most progressive childhood neuromuscular disorders. It affects mostly boys, but occasionally girls are affected. According to Muscular Dystrophy Australia, in 2016, Duchenne…
