Press release
Electronic Trial Master File (eTMF) Systems Market: An Overview
Introduction:The Electronic Trial Master File (eTMF) system is a digital platform used to store and manage essential documents related to clinical trials. It helps organizations ensure regulatory compliance, streamline operations, and improve collaboration among stakeholders such as sponsors, clinical research organizations (CROs), and regulatory bodies. eTMF systems replace paper-based filing systems, offering a more efficient way to track, manage, and store trial documents. With the increasing number of clinical trials and the shift toward digitization in the life sciences sector, the demand for eTMF systems has grown substantially.
Market Size:
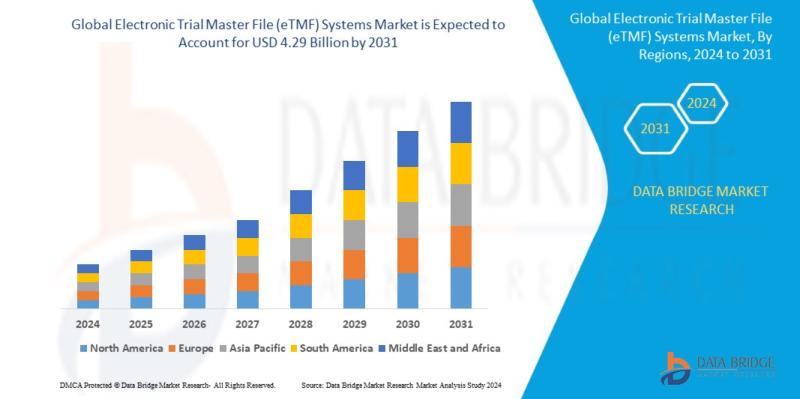
Global electronic trial master file (ETMF) systems market size was valued at USD 1.63 billion in 2023 and is projected to reach USD 4.29 billion by 2031, with a CAGR of 12.9% during the forecast period of 2024 to 2031. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, geographically represented company-wise production and capacity, network layouts of distributors and partners, detailed and updated price trend analysis and deficit analysis of supply chain and demand.
https://www.databridgemarketresearch.com/reports/global-electronic-trial-master-file-etmf-systems-market
Some of the major players operating in the market are:
IQVIA Inc.(U.S.)
Labcorp Drug Development (U.S.)
TransPerfect (U.S.)
Oracle (U.S.)
Phlexglobal (U.S.)
SureClinical Inc. (U.S.)
Aurea, Inc. (U.S.)
Veeva Systems (U.S.)
MasterControl Solutions, Inc. (U.S.)
Clinevo Technologies (India)
Mayo Foundation for Medical Education and Research (MFMER) (U.S.)
Montrium Inc. (U.S.)
NCGD Inc. (U.S.)
PharmaVigilance (U.S.)
Market Share:
The eTMF systems market is dominated by a few major players, with Veeva Systems being a leading provider, holding a significant market share. Other key players include Oracle Corporation, Phlexglobal, TransPerfect, and Medidata Solutions. These companies offer advanced eTMF systems that enable organizations to automate document management processes, ensuring compliance with regulatory requirements such as Good Clinical Practice (GCP) guidelines and FDA regulations.
Pharmaceutical and biotechnology companies are the largest users of eTMF systems, accounting for more than 50% of the market share. Contract research organizations (CROs) and regulatory agencies also contribute significantly to the market, as they increasingly rely on eTMF systems to manage clinical trial documentation and streamline workflows.
Market Trends:
Several key trends are shaping the eTMF systems market. One of the most prominent trends is the growing adoption of cloud-based eTMF systems. Cloud-based platforms offer greater flexibility, scalability, and accessibility, allowing organizations to store and manage large volumes of clinical trial documents securely. These systems also facilitate real-time collaboration among global teams, improving efficiency and reducing the time required to complete clinical trials.
Another notable trend is the integration of eTMF systems with other clinical trial management tools. Many organizations are seeking to create unified platforms that streamline the entire clinical trial process, from study planning and site selection to document management and regulatory submissions. This integration helps organizations improve data accuracy, reduce duplication of effort, and ensure that all trial-related documents are easily accessible and up-to-date.
The use of artificial intelligence (AI) and machine learning (ML) in eTMF systems is also gaining traction. AI-powered eTMF systems can automate document classification, indexing, and quality control processes, reducing manual effort and improving compliance. These advanced technologies also help organizations identify potential risks and ensure that trial documents meet regulatory requirements.
The increasing focus on data security and compliance is another important trend. With the growing volume of sensitive patient data being stored in eTMF systems, organizations are investing in advanced security measures such as encryption, multi-factor authentication, and access controls to protect clinical trial documents from unauthorized access and breaches.
Market Growth:
The eTMF systems market is experiencing strong growth, driven by several factors. One of the primary drivers is the increasing number of clinical trials being conducted globally. As the demand for new therapies and drugs continues to rise, pharmaceutical and biotechnology companies are expanding their clinical trial activities. This growth in clinical trials is creating a need for efficient and compliant document management systems, driving the adoption of eTMF systems.
The shift toward digitization in the life sciences sector is also contributing to market growth. Many organizations are transitioning from paper-based filing systems to digital solutions to improve efficiency, reduce costs, and ensure compliance with regulatory requirements. eTMF systems offer a streamlined way to manage clinical trial documents, reducing the risk of errors and ensuring that all documents are easily accessible for audits and inspections.
Another factor driving market growth is the increasing emphasis on regulatory compliance. Regulatory agencies such as the FDA and the European Medicines Agency (EMA) have strict requirements for clinical trial documentation. Organizations are adopting eTMF systems to ensure that their trial documents meet these regulatory requirements and can be easily accessed during audits.
The growing demand for remote clinical trials and decentralized trial models is also fueling the growth of the eTMF systems market. As more clinical trials are conducted remotely, organizations need digital platforms to manage trial documents and ensure that all stakeholders can access the necessary information in real-time.
Market Demand:
The demand for eTMF systems is being driven by several key factors. One of the most significant is the increasing complexity of clinical trials. As trials become more complex, organizations need efficient systems to manage the large volumes of documents and ensure compliance with regulatory requirements. eTMF systems provide a solution to this challenge, offering a centralized platform for document management and collaboration.
The growing number of global clinical trials is also driving demand for eTMF systems. Many pharmaceutical and biotechnology companies are conducting trials across multiple countries, requiring systems that can facilitate collaboration among global teams. eTMF systems enable real-time access to trial documents, ensuring that all stakeholders are on the same page and can contribute to the trial's success.
The need for faster clinical trial timelines is another factor driving demand for eTMF systems. Organizations are under pressure to bring new therapies and drugs to market as quickly as possible. eTMF systems help streamline the document management process, reducing the time required to prepare and submit regulatory documents and improving overall trial efficiency.
The rising adoption of decentralized clinical trials (DCTs) is also contributing to the demand for eTMF systems. DCTs involve remote data collection and monitoring, making it essential for organizations to have digital platforms in place to manage trial documents and ensure regulatory compliance.
Factors Driving Growth:
Several factors are driving the growth of the eTMF systems market. One of the key drivers is the increasing focus on regulatory compliance. Organizations must ensure that their clinical trial documents meet regulatory requirements and are easily accessible for audits and inspections. eTMF systems provide a solution to this challenge, offering automated workflows, version control, and audit trails that help organizations stay compliant with GCP guidelines and FDA regulations.
Technological advancements are also driving growth in the market. The development of AI-powered eTMF systems is improving the efficiency of document management processes, reducing the need for manual intervention, and ensuring that trial documents meet regulatory requirements. These advanced systems are also helping organizations identify potential risks and ensure that trial documents are of high quality.
The rising demand for cloud-based solutions is another factor driving market growth. Cloud-based eTMF systems offer greater flexibility, scalability, and accessibility, making them an attractive option for organizations conducting global clinical trials. These systems also provide enhanced security features, ensuring that sensitive patient data is protected.
Finally, the increasing number of clinical trials being conducted worldwide is driving growth in the eTMF systems market. As pharmaceutical and biotechnology companies expand their trial activities, the need for efficient and compliant document management systems is growing, leading to increased adoption of eTMF systems.
Conclusion:
The Electronic Trial Master File (eTMF) systems market is experiencing robust growth, driven by the increasing number of clinical trials, the shift toward digitization, and the growing emphasis on regulatory compliance. With technological advancements such as AI integration, cloud-based platforms, and enhanced security features, eTMF systems are becoming an essential tool for managing clinical trial documents. As the market continues to grow, organizations in the life sciences sector will increasingly rely on eTMF systems to ensure the success of their clinical trials and meet regulatory requirements.
Browse Trending Reports:
https://xingsh.blogspot.com/2024/09/central-fill-pharmacy-automation-market.html
https://xingsh.blogspot.com/2024/09/embedded-connectivity-solutions-market.html
https://xingsh.blogspot.com/2024/09/cathode-materials-market-size-share.html
https://xingsh.blogspot.com/2024/09/industrial-cooking-fire-protection.html
Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC : +653 1251 975
Email: corporatesales@databridgemarketresearch.com
About Data Bridge Market Research:
Data Bridge set forth itself as an unconventional and neoteric Market research and consulting firm with unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Electronic Trial Master File (eTMF) Systems Market: An Overview here
News-ID: 3662272 • Views: …
More Releases from Data Bridge Market Research
Hemodialysis and Peritoneal Dialysis Market: An Overview
Introduction:
The Hemodialysis and Peritoneal Dialysis market is essential for treating chronic kidney disease (CKD) and end-stage renal disease (ESRD). These dialysis methods offer lifesaving options for patients whose kidneys can no longer function adequately. Hemodialysis uses a machine and a dialyzer to filter waste and excess fluids from the blood, while peritoneal dialysis uses the lining of the abdomen to perform this function at home. With the rising global prevalence…
Midline Catheter Market
The midline catheter market plays a crucial role in healthcare, providing a safer and more effective means for intravenous (IV) therapies. Midline catheters are long, thin, flexible tubes that are inserted into a patient's veins, typically for periods ranging from a few days to a few weeks. They offer a minimally invasive alternative for administering medications, fluids, and nutrients directly into the bloodstream without the need for repeated needle sticks.…
Microinsurance Market
Microinsurance refers to insurance products designed to be affordable and accessible to low-income individuals or those who are typically underserved by traditional insurance markets. It offers coverage for various risks, such as health, life, property, and agriculture, but with smaller premiums and lower coverage limits. Microinsurance is especially important in developing countries, where people face significant risks but lack the financial means to access conventional insurance products. The primary aim…
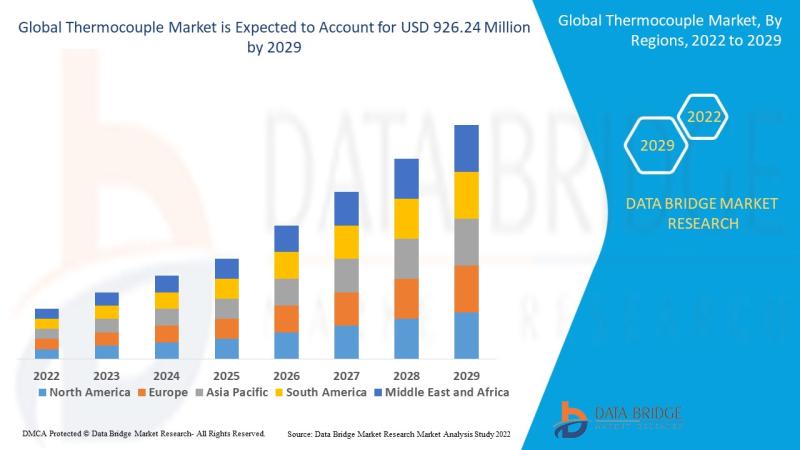
Thermocouple Market
A thermocouple is a type of temperature sensor that plays a vital role in various industries. It works by converting thermal energy into electrical energy, which can then be used to measure temperature. Thermocouples are widely used because of their simplicity, wide temperature range, durability, and affordability. Their versatility makes them applicable across industries like automotive, aerospace, power generation, manufacturing, and healthcare. They are used to monitor and control temperatures…
More Releases for Master
Performance of a great master
Havana, February 21, 2020 - The researcher in charge of the project "Fidel Mic� Catalogue Raisonn�" is pleased to announce the public performance of the great master from February 21 to 28. Mic� will be painting at the private gallery "Humidores Habana" (Bulevar de San Rafael, Centro Habana, La Habana, Cuba) to show his exquisite technique to create high quality landscape paintings. Access will be free immediately after inauguration ceremony…
MASTER CHEFS DINNER
Nusa Dua, Bali, Indonesia, September 2017… Food lovers are in for a real treat as four Master Chefs will be joining forces at The Westin Resort Nusa Dua and Bali Convention Centre, Bali on Saturday, 7th October 2017 for an evening of flavor and flair. This curated dining event promises to please palates with a vibrant four-course menu and a selection of corresponding wines. Each dish will be prepared at…
Master Data Management Market: Master Data Management Investments in BFSI Sector …
As the amount of data generated by organizations multiples rapidly, organizations can no longer afford to delay investments in master data management solutions. This will help the opportunity in the global master data management market to spiral to US$37.97 bn by 2024, rising at a 27.25% CAGR from 2016 to 2024. The market had a valuation of US$4.35 bn in 2015. Consolidation among companies is helping this market blaze ahead,…
Global Master Control Switchers Market
Key players in the master control switchers market include Evertz Microsystems, Ltd, Harris Corporation, Grass Valley USA, LLC, PESA, Miranda Technologies, Inc., Pixel Power, Inc., Utah Scientific, Inc and Snell Group.
Master control switchers are an essential part of television and video broadcast that aggregate programming feeds from different sources such as audio and video. Global emergence of broadcasting industry forced operational cost reduction and technological enhancements in the same,…
Master Equipment Appraisers now has 5 Master Certified Machinery & Equipment App …
Master Equipment Appraisers, a reputable appraisal service provider unveiled that they now have 5 of the 23 master certified machinery and equipment appraisers in the US. Having such industry-best appraisers, the company offers the best appraisals one could ever get in the area.
Master Equipment Appraisers is a leading firm offering marketing and consulting services such as business buy/sell brokerage, commercial real estate appraisal, business valuation and equipment appraisal services. They…
EtherCAT Master Stack
IXXAT now offers an EtherCAT Master Stack, specially suited for the usage under real time operating systems on embedded platforms. At this, many conventional network interface cards are directly supported, so no special hardware is required for the Ethernet connection.
Already late last year, IXXAT extended its EtherCAT product portfolio by establishing a cooperation with acontis technologies GmbH, a leading technology provider in the area of…