Press release
e-Clinical Solutions Market: An In-Depth Analysis
Introduction:The e-Clinical solutions market encompasses a wide range of software and services that assist in the management and automation of clinical trials. These solutions facilitate the electronic collection, analysis, and management of clinical data, significantly improving the efficiency and accuracy of clinical trials. With the increasing complexity of clinical trials and the need for faster drug development, e-Clinical solutions have become an essential tool for pharmaceutical companies, contract research organizations (CROs), and other stakeholders involved in clinical research. These solutions include electronic data capture (EDC), clinical trial management systems (CTMS), randomization and trial supply management (RTSM), and other software aimed at streamlining the clinical trial process.
Market Size:
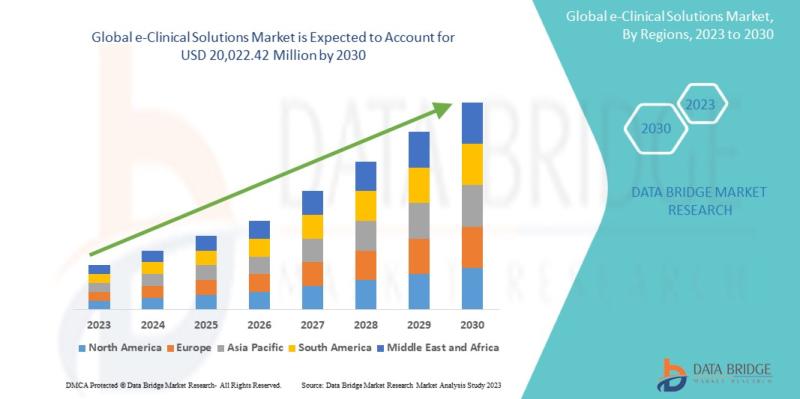
Data Bridge Market Research analyzes that the global e-clinical solutions market is expected to reach the value of USD 20,022.42 million by 2030, at a CAGR of 13.5% during the forecast period. This market report also covers pricing analysis and technological advancements in depth.
https://www.databridgemarketresearch.com/reports/global-eclinical-solutions-market
Some of the major market players operating in the global e-clinical solutions market are Oracle, Signant Health, MaxisIT, Paraxel International Corporation, Dassault Systemes, Clario, Mednet, OpenClinica, LLC, 4G Clinical, Veeva Systems, Saama Technologies, LLC, Anju, Castor, Medrio, Inc., ArisGlobal, Merative, Advarra, eClinical Solutions, LLC, Y-Prime LLC, and RealTime Software Solutions LLC among others.
Market Share:
The e-Clinical solutions market is highly competitive, with several key players dominating the industry. Companies such as Oracle Corporation, Medidata Solutions (a Dassault Systèmes company), PAREXEL International Corporation, and Veeva Systems hold significant market shares due to their comprehensive product portfolios and strong presence in the global market. Oracle Corporation and Medidata Solutions are particularly notable for their extensive range of e-Clinical solutions, covering various aspects of clinical trial management and data analysis.
Geographically, North America holds the largest market share, accounting for over 40% of the global e-Clinical solutions market. This dominance is primarily due to the presence of a large number of pharmaceutical and biotechnology companies in the region, as well as the high adoption of advanced technologies in clinical research. Europe and the Asia-Pacific region also hold significant market shares, driven by the increasing number of clinical trials and growing investments in healthcare infrastructure.
Market Trends:
Several key trends are shaping the e-Clinical solutions market. One of the most notable trends is the increasing adoption of cloud-based e-Clinical solutions. Cloud-based solutions offer several advantages over traditional on-premise systems, including scalability, cost-effectiveness, and remote access. The ability to access clinical trial data from anywhere in the world, coupled with the reduced need for IT infrastructure, has made cloud-based e-Clinical solutions increasingly popular among pharmaceutical companies and CROs.
Another significant trend is the integration of artificial intelligence (AI) and machine learning (ML) into e-Clinical solutions. AI and ML technologies are being used to analyze large datasets, identify patterns, and make predictions, ultimately improving the efficiency and accuracy of clinical trials. For example, AI-powered analytics can help identify potential risks in clinical trials, optimize patient recruitment, and enhance data quality. The increasing use of AI and ML in e-Clinical solutions is expected to drive innovation and improve the overall effectiveness of clinical trials.
The growing emphasis on patient-centric clinical trials is also shaping the e-Clinical solutions market. There is a shift towards more patient-friendly trial designs that prioritize the needs and preferences of patients. This trend is driving the development of e-Clinical solutions that enable remote monitoring, virtual trials, and patient-reported outcomes. These solutions allow patients to participate in clinical trials from the comfort of their homes, reducing the burden of travel and increasing patient engagement and retention.
Market Growth:
The e-Clinical solutions market is expected to experience robust growth in the coming years, driven by several factors. The increasing complexity of clinical trials is a primary driver of market growth. As clinical trials become more intricate, with larger sample sizes, multiple endpoints, and diverse patient populations, the need for efficient data management and analysis becomes more critical. e-Clinical solutions provide the tools necessary to manage this complexity, ensuring that clinical trials are conducted efficiently and accurately.
The growing demand for faster drug development is also contributing to the growth of the e-Clinical solutions market. Pharmaceutical companies are under increasing pressure to bring new drugs to market more quickly, particularly in the face of rising healthcare costs and the need for innovative treatments. e-Clinical solutions enable companies to streamline the clinical trial process, reduce timelines, and accelerate drug development. The increasing adoption of these solutions is expected to drive market growth.
The rising adoption of digital health technologies is another factor fueling market growth. The integration of e-Clinical solutions with other digital health tools, such as electronic health records (EHRs) and telemedicine platforms, is making it easier to collect and analyze clinical trial data. The growing use of digital health technologies is expected to drive the adoption of e-Clinical solutions, further contributing to market growth.
Moreover, the increasing focus on regulatory compliance is driving demand for e-Clinical solutions. Regulatory agencies, such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA), have stringent requirements for the collection, storage, and analysis of clinical trial data. e-Clinical solutions help ensure that clinical trials comply with these regulations, reducing the risk of non-compliance and associated penalties.
Market Demand:
The demand for e-Clinical solutions is growing rapidly, driven by several factors. One of the primary drivers of demand is the increasing number of clinical trials. The global pharmaceutical and biotechnology industries are experiencing a surge in clinical trial activity, driven by the need for new treatments and therapies. As the number of clinical trials increases, so does the demand for efficient data management and analysis tools, driving the adoption of e-Clinical solutions.
The growing trend towards personalized medicine is also contributing to the demand for e-Clinical solutions. Personalized medicine involves tailoring treatments to individual patients based on their genetic makeup, lifestyle, and other factors. This approach requires the collection and analysis of large amounts of data, which can be efficiently managed using e-Clinical solutions. The increasing focus on personalized medicine is expected to drive the demand for e-Clinical solutions in the coming years.
The rise of decentralized and virtual clinical trials is another factor driving demand for e-Clinical solutions. Decentralized trials involve conducting clinical trials at multiple locations, often with patients participating remotely. Virtual trials, on the other hand, are conducted entirely online, with no need for physical visits to clinical trial sites. Both decentralized and virtual trials require robust e-Clinical solutions to manage data collection, monitoring, and analysis, driving demand for these solutions.
The growing emphasis on data security and privacy is also contributing to the demand for e-Clinical solutions. With the increasing amount of data being collected in clinical trials, there is a growing need for secure and compliant data management solutions. e-Clinical solutions offer robust data security features, ensuring that clinical trial data is protected from unauthorized access and breaches. The increasing focus on data security and privacy is expected to drive the demand for e-Clinical solutions.
Factors Driving Growth:
Several factors are driving the growth of the e-Clinical solutions market. The increasing complexity of clinical trials is one of the most significant factors. As clinical trials become more intricate, the need for efficient data management and analysis becomes more critical. e-Clinical solutions provide the tools necessary to manage this complexity, ensuring that clinical trials are conducted efficiently and accurately.
The growing demand for faster drug development is another key driver of market growth. Pharmaceutical companies are under increasing pressure to bring new drugs to market more quickly, particularly in the face of rising healthcare costs and the need for innovative treatments. e-Clinical solutions enable companies to streamline the clinical trial process, reduce timelines, and accelerate drug development. The increasing adoption of these solutions is expected to drive market growth.
The rising adoption of digital health technologies is also contributing to market growth. The integration of e-Clinical solutions with other digital health tools, such as EHRs and telemedicine platforms, is making it easier to collect and analyze clinical trial data. The growing use of digital health technologies is expected to drive the adoption of e-Clinical solutions, further contributing to market growth.
The increasing focus on regulatory compliance is another factor driving demand for e-Clinical solutions. Regulatory agencies have stringent requirements for the collection, storage, and analysis of clinical trial data. e-Clinical solutions help ensure that clinical trials comply with these regulations, reducing the risk of non-compliance and associated penalties.
Finally, the growing emphasis on patient-centric clinical trials is driving the development of e-Clinical solutions that enable remote monitoring, virtual trials, and patient-reported outcomes. These solutions allow patients to participate in clinical trials from the comfort of their homes, reducing the burden of travel and increasing patient engagement and retention.
Conclusion:
The e-Clinical solutions market is poised for significant growth in the coming years, driven by increasing clinical trial complexity, growing demand for faster drug development, and the rising adoption of digital health technologies. As the market continues to evolve, key trends such as the integration of AI and ML, the shift towards patient-centric trials, and the adoption of cloud-based solutions are expected to shape the future of the industry. With ongoing advancements in technology and increasing regulatory requirements, the e-Clinical solutions market offers
significant opportunities for growth and innovation. As the demand for efficient clinical trial management continues to rise, the e-Clinical solutions market is well-positioned to meet the needs of the global healthcare industry.
Browse Trending Reports:
https://o9sep.blogspot.com/2024/09/coal-to-liquid-market-size-share-trends.html
https://o9sep.blogspot.com/2024/09/forage-harvester-market-size-share.html
https://o9sep.blogspot.com/2024/09/micro-and-nano-programmable-logic.html
https://o9sep.blogspot.com/2024/09/erythropoietin-epo-drugs-market-size.html
Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC : +653 1251 975
Email: corporatesales@databridgemarketresearch.com
About Data Bridge Market Research:
Data Bridge set forth itself as an unconventional and neoteric Market research and consulting firm with unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release e-Clinical Solutions Market: An In-Depth Analysis here
News-ID: 3649674 • Views: …
More Releases from Data Bridge Market Research
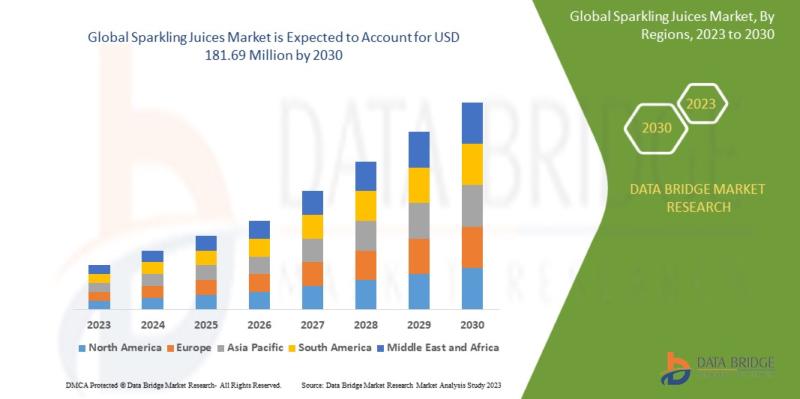
Sparkling Juices Market to Observe Prominent CAGR of 8.5% by 2030, Size, Share, …
Data Bridge Market Research analyses the market is expected to reach USD 181.69 million by 2030 from 94.60 million in 2022 growing at a CAGR of 8.5 % in the sparkling Juices market.
Market Definition:
Sparkling juice is a non-alcoholic beverage made by carbonating fruit juice. It typically contains real fruit juice, carbonated water, and sweeteners. The carbonation gives it a fizzy, effervescent quality, similar to soda or champagne, without the alcohol.…
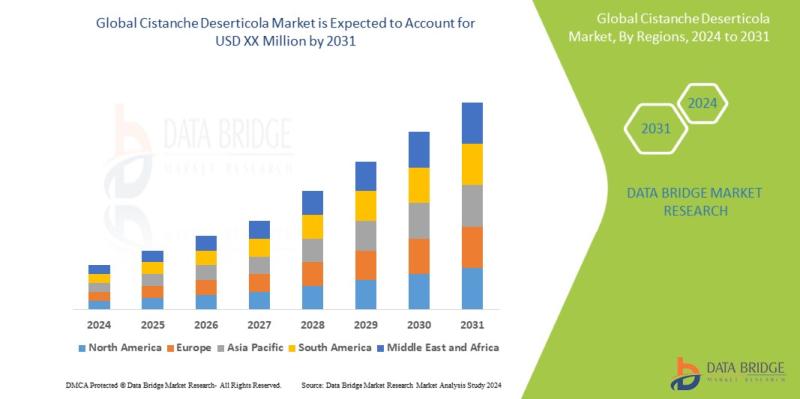
Cistanche Deserticola Market to Observe Prominent CAGR of 5.6% by 2031, Size, Sh …
Data Bridge Market Research analyzes that the cistanche deserticola market is expected to undergo a CAGR of 5.6% during the forecast period. "Knowledge-Based" dominates the type segment of the Cistanche deserticola market owing to the growing demand for better methods for treatment in patients.
Market Definition:
Cistanche deserticola is one of the endangered herbal species founded in arid and semi-arid conditions. This species is extensively used for treatment of several kinds of…
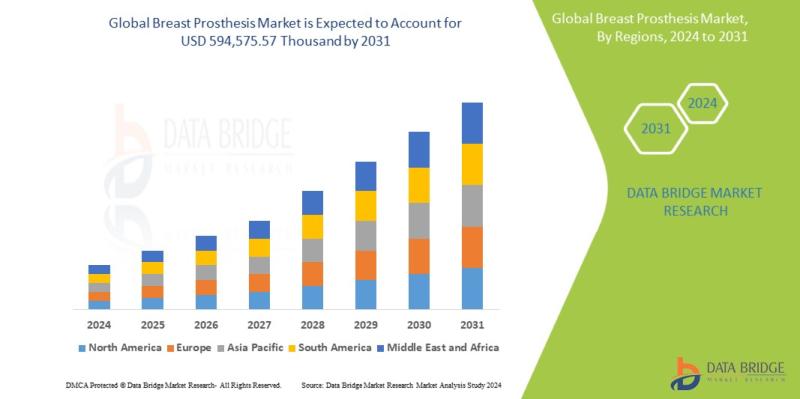
Breast Prosthesis Market to Observe Prominent CAGR of 8.3% by 2031, Size, Share, …
Data Bridge Market Research analyzes that the global breast prosthesis market is expected to reach USD 594,575.57 thousand by 2031 from USD 319,959.41 thousand million in 2023, growing at a CAGR of 8.3% in the forecast period of 2024 to 2031.
Market Definition:
A breast prosthesis is an artificial breast form designed to replace all or part of a natural breast that has been removed due to mastectomy, lumpectomy, or other medical…
Exploring the Aesthetic Devices Market: An In-Depth Analysis 2029
The aesthetic devices market has evolved significantly, driven by advancements in technology and growing consumer demand for non-invasive cosmetic procedures. Aesthetic devices encompass a range of equipment used for enhancing physical appearance, including lasers, radiofrequency devices, ultrasound machines, and more. These devices cater to various cosmetic needs, from skin rejuvenation to body contouring. This post provides a comprehensive overview of the aesthetic devices market, detailing its scope, exploring current market…
More Releases for Clinical
Clinical Laboratory Market in Indonesia, Clinical Laboratory Industry in Indones …
"Increase in healthcare expenditure from the Indonesian government has driven the growth of clinical laboratory market in Indonesia."
Increase in Healthcare Awareness: Largely driven by increase in healthcare spending by aging population (~$ 260 per person by 2050), rising income levels, rising awareness for preventive testing, advanced healthcare diagnostic tests offerings, and central government's healthcare measures.
Developments in Testing and Preference for Evidence based testing: There is also a rising number…
Clinical solutions
Are you spending more time with yellow files than with patients? Healthbridge can change that with our intuitive and easy to use clinical platform that is designed specifically for the medical practitioner at the practice.
smaller2
Easily access patient
information
Cloud-based technology enables you to store rich clinical information that can be easily accessed as and when you need it.
Medical billing software innovation
Become a paperless
practice
Create scripts, sick notes, and clinical notes electronically. Plus, have…
Clinical Research and Clinical Trials Summit
Clinical Research 2019 has been designed in an interdisciplinary manner with a multitude of tracks to choose from every segment and provides you with a unique opportunity to meet up with peers from both industry and academia and establish a scientific network between them. We cordially invite all concerned people to come join us at our event and make it successful by your participation.
This is the premier interdisciplinary forum for…
Paediatric Clinical Trial Conference - When designing a Paediatric clinical tria …
Press Release – 12.02.2018
When designing a Paediatric clinical trial, a paediatric investigation plan (PIP) is a development plan aimed at ensuring that the necessary data are obtained through studies in children, to support the authorisation of a medicine for children. All applications for marketing authorisation for new medicines have to include the results of studies as described in an agreed PIP, unless the medicine is exempt because of a deferral…
Clinical Communication
According to a recent market report published by Persistence Market Research titled “Clinical Communication and Collaboration Market: Global Industry Analysis (2012–2016) and Forecast (2017–2025),” revenue from the global clinical communication and collaboration market was US$ 138.5 Mn in 2012 and US$ 214.8 Mn in 2016, representing a CAGR of 11.6% from 2012 to 2016. This revenue growth is attributed to addition of new features in clinical communication and collaboration solutions.…
E-Clinical Trial Solutions Market To Accelerating Clinical Development Technolog …
The study of the "Global e-Clinical Trial Solutions Market" provides the market size information and market trends along with the factors and parameters impacting it in both short and long term. The study ensures a 360° view, bringing out the complete key insights of the industry.
The Global e-Clinical Trial Solutions Market Research Report Forecast 2017-2021 is a valuable source of insightful data for business strategists. It provides the e-Clinical…