Press release
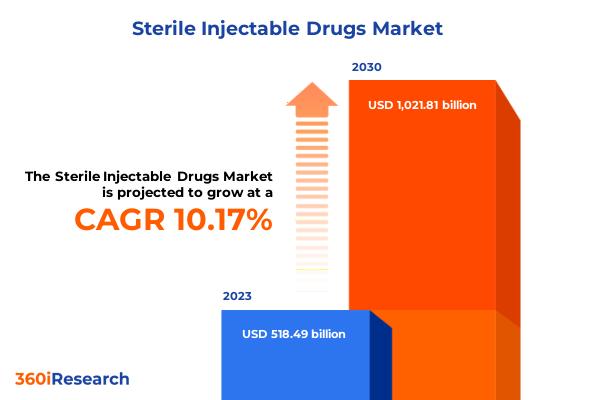
Sterile Injectable Drugs Market worth $1,021.81 billion by 2030, growing at a CAGR of 10.17% - Exclusive Report by 360iResearch
The "Sterile Injectable Drugs Market by Molecule Type (Large Molecule, Small Molecule), Drug (Blood Factors, Cytokines, Immunoglobulins), Indication, Distribution - Global Forecast 2024-2030" report has been added to 360iResearch.com's offering.Request a Free Sample Report @ https://www.360iresearch.com/library/intelligence/sterile-injectable-drugs?utm_source=openpr&utm_medium=referral&utm_campaign=sample
"Growing Demand for Sterile Injectable Drugs Amidst Rising Chronic Diseases and Enhanced Regulatory Support"
The increasing global prevalence of chronic diseases such as diabetes, cancer, and cardiovascular conditions, coupled with urgent healthcare needs, is significantly driving the demand for sterile injectable drugs. These medications play a crucial role in a range of therapeutic areas, from treating infectious diseases to managing pain, offering a cost-effective and immediate solution in various healthcare settings, including hospitals and specialty clinics. Moreover, the pharmaceutical landscape is witnessing streamlined regulatory processes that facilitate quicker market access for these drugs, notably those addressing severe health conditions. Economic efficiency, ease of use, and effectiveness of sterile injectables compared to more invasive treatments further elevate their preference in resource-limited environments. Additionally, the market is expanding through strategic partnerships and reforms that aim to enhance drug availability and affordability, ultimately boosting global accessibility to essential healthcare solutions.
"Navigating Challenges in the Sterile Injectable Drugs Market: Supply, Quality, and Innovation Barriers"
The production of sterile injectable drugs is crucially dependent on the consistent availability and quality of raw materials, with any compromise likely leading to production delays, higher costs, and risks to drug efficacy and safety. Additionally, these drugs must adhere to stringent standards to prevent issues like pH imbalances and particulate contamination, which can cause costly recalls and damage both reputation and consumer trust. The sector also faces high entry and operational costs due to its reliance on advanced technology and strict regulatory compliance, barriers that can stifle new competitors and constrain expansion. Globally, varying standards create additional hurdles, complicating distribution and market access. Moreover, the rise of alternative delivery technologies such as transdermal patches and oral biologics presents new competition, potentially diverting demand from injectable solutions. Together, these factors pose significant challenges for manufacturers in the sterile injectables market.
"Revolutionizing Sterile Injectable Drugs: Innovative Technologies and Strategic Partnerships Propel Market Growth"
In an era of rapid medical advancements, the sterile injectable drugs sector is witnessing transformative changes, with significant improvements in production technologies that enhance capacity and reduce costs. This evolution enables manufacturers to better satisfy the escalating global demand. Furthermore, the industry is capitalizing on personalized medicine, particularly in oncology and chronic illnesses, to offer treatments tailored to individual genetic profiles, thus enhancing patient outcomes and minimizing adverse reactions. Regulatory reforms are also crucial, as streamlined approval processes allow quicker market entry of new and generic injectables, boosting the pharmaceutical industry's responsiveness to public health challenges. Collaborative efforts in research and development among corporations, academic entities, and labs are driving forward drug effectiveness and versatility. Additionally, investments in startups focusing on cutting-edge drug delivery technologies promise to invigorate the sector with safer and more precise treatments. Enhancements in packaging are extending the shelf life and stability of products, ensuring quality throughout distribution and storage. Lastly, public-private partnerships are vital in developing infrastructure in emerging markets, facilitating local production capabilities, and expanding global access to essential medications. These concerted efforts are not only enhancing the market dynamics but are also aligned with broader global health objectives.
"Navigating Challenges in the Sterile Injectable Drug Industry: From Production to Market Expansion"
The development and manufacturing of sterile injectable drugs demand adherence to stringent regulatory standards to ensure the safety and potency of these vital health treatments. The complexity of maintaining a contamination-free environment requires advanced technological facilities, which significantly drives up both time and costs. Besides facing severe risks from counterfeit drugs that compromise patient safety and confidence, the industry also contends with environmentally detrimental impacts during production and disposal phases. As this sector emerges in new global markets, it confronts varied regulatory landscapes that delay market entry and inflate costs, while grappling with the additional challenge of setting up reliable logistics for fragile products. Moreover, there is an urgent need to bridge the gap in the skilled workforce, essential to uphold manufacturing quality and compliance, thus ensuring a continuous supply of critical medications. This press release aims to shed light on these multifaceted challenges, emphasizing the industry's commitment to overcoming them to ensure effective healthcare solutions reach those in need safely and sustainably.
Inquire Before Buying @ https://www.360iresearch.com/library/intelligence/sterile-injectable-drugs?utm_source=openpr&utm_medium=referral&utm_campaign=inquire
Market Segmentation & Coverage:
This research report categorizes the Sterile Injectable Drugs Market in order to forecast the revenues and analyze trends in each of following sub-markets:
Based on Molecule Type, market is studied across Large Molecule and Small Molecule.
Based on Drug, market is studied across Blood Factors, Cytokines, Immunoglobulins, Insulin, Monoclonal Antibodies (mAbs), Peptide Hormones, and Vaccine.
Based on Indication, market is studied across Autoimmune Diseases, Cardiovascular & Metabolic Diseases, Gastroenterology, Hematology, Infectious Diseases, Neurology, and Oncology.
Based on Distribution, market is studied across E-commerce, Hospital Pharmacy, and Retail Pharmacy.
Based on Region, market is studied across Americas, Asia-Pacific, and Europe, Middle East & Africa. The Americas is further studied across Argentina, Brazil, Canada, Mexico, and United States. The United States is further studied across California, Florida, Illinois, New York, Ohio, Pennsylvania, and Texas. The Asia-Pacific is further studied across Australia, China, India, Indonesia, Japan, Malaysia, Philippines, Singapore, South Korea, Taiwan, Thailand, and Vietnam. The Europe, Middle East & Africa is further studied across Denmark, Egypt, Finland, France, Germany, Israel, Italy, Netherlands, Nigeria, Norway, Poland, Qatar, Russia, Saudi Arabia, South Africa, Spain, Sweden, Switzerland, Turkey, United Arab Emirates, and United Kingdom.
Key Company Profiles:
The report delves into recent significant developments in the Sterile Injectable Drugs Market, highlighting leading vendors and their innovative profiles. These include Adare Pharma Solutions, Aenova Group, Almac Group, Avara Pharmaceutical Services, Inc., Baxter International Inc., Boehringer Ingelheim International GmbH, Catalent, Inc., CordenPharma, Eli Lilly and Company, Evonik Industries AG, F. Hoffmann-La Roche Ltd, FAMAR Health Care Services, Fresenius Kabi Ag, Grifols S.A., Hikma Pharmaceuticals PLC, Jubilant Pharmova Limited, Lonza, Nexus Pharmaceuticals, Inc., Patheon, Inc. (Thermo Fischer), Pfizer Inc., Recipharm AB, and Siegfried Holding AG.
Introducing ThinkMi Query: Revolutionizing Market Intelligence with AI-Powered Insights for the Sterile Injectable Drugs Market
We proudly unveil ThinkMi Query, a cutting-edge AI product designed to transform how businesses interact with the Sterile Injectable Drugs Market. ThinkMi Query stands out as your premier market intelligence partner, delivering unparalleled insights with the power of artificial intelligence. Whether deciphering market trends or offering actionable intelligence, ThinkMi Query is engineered to provide precise, relevant answers to your most critical business questions. This revolutionary tool is more than just an information source; it's a strategic asset that empowers your decision-making with up-to-the-minute data, ensuring you stay ahead in the fiercely competitive Sterile Injectable Drugs Market. Embrace the future of market analysis with ThinkMi Query, where informed decisions lead to remarkable growth.
Ask Question to ThinkMi Query @ https://www.360iresearch.com/library/intelligence/sterile-injectable-drugs?utm_source=openpr&utm_medium=referral&utm_campaign=query
Key Topics Covered:
1. Preface
2. Research Methodology
3. Executive Summary
4. Market Overview
5. Market Insights
6. Sterile Injectable Drugs Market, by Molecule Type
7. Sterile Injectable Drugs Market, by Drug
8. Sterile Injectable Drugs Market, by Indication
9. Sterile Injectable Drugs Market, by Distribution
10. Americas Sterile Injectable Drugs Market
11. Asia-Pacific Sterile Injectable Drugs Market
12. Europe, Middle East & Africa Sterile Injectable Drugs Market
13. Competitive Landscape
14. Competitive Portfolio
Read More @ https://www.360iresearch.com/library/intelligence/sterile-injectable-drugs?utm_source=openpr&utm_medium=referral&utm_campaign=analyst
Contact 360iResearch
Mr. Ketan Rohom
Sales & Marketing,
Office No. 519, Nyati Empress,
Opposite Phoenix Market City,
Vimannagar, Pune, Maharashtra,
India - 411014.
sales@360iresearch.com
+1-530-264-8485
+91-922-607-7550
About 360iResearch
360iResearch is a market research and business consulting company headquartered in India, with clients and focus markets spanning the globe.
We are a dynamic, nimble company that believes in carving ambitious, purposeful goals and achieving them with the backing of our greatest asset - our people.
Quick on our feet, we have our ear to the ground when it comes to market intelligence and volatility. Our market intelligence is diligent, real-time and tailored to your needs, and arms you with all the insight that empowers strategic decision-making.
Our clientele encompasses about 80% of the Fortune Global 500, and leading consulting and research companies and academic institutions that rely on our expertise in compiling data in niche markets. Our meta-insights are intelligent, impactful and infinite, and translate into actionable data that support your quest for enhanced profitability, tapping into niche markets, and exploring new revenue opportunities.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Sterile Injectable Drugs Market worth $1,021.81 billion by 2030, growing at a CAGR of 10.17% - Exclusive Report by 360iResearch here
News-ID: 3516737 • Views: …
More Releases from 360iResearch
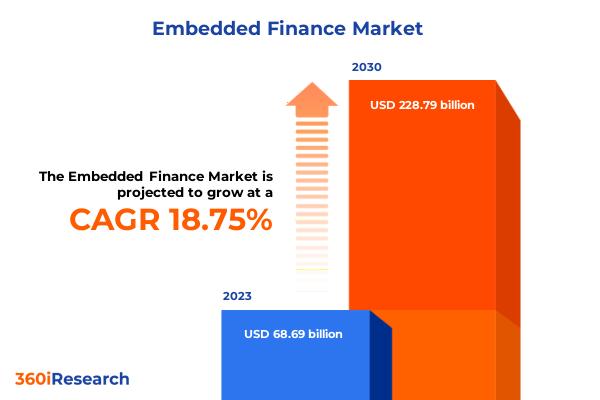
Embedded Finance Market worth $228.79 billion by 2030, growing at a CAGR of 18.7 …
The "Embedded Finance Market by Services (Embedded Banking Services, Embedded Insurance, Embedded Lending), Integration Type (Application Programming Interfaces, Software Development Kits), Business Model, End-Use - Global Forecast 2024-2030" report has been added to 360iResearch.com's offering.
Request a Free Sample Report @ https://www.360iresearch.com/library/intelligence/embedded-finance
Embedded Finance refers to the integration of financial services, such as lending, insurance, payments, or banking, directly within the products or services of non-financial companies using Application Programming Interfaces (APIs).…
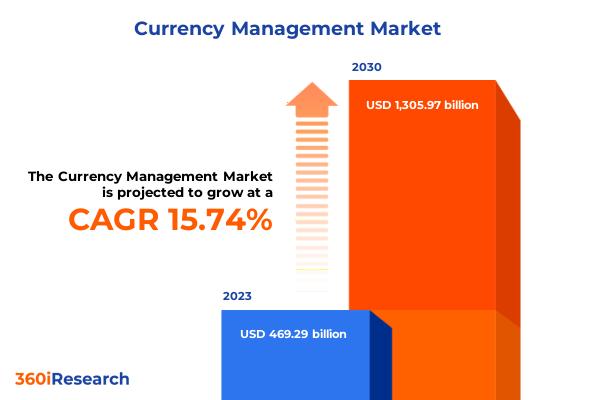
Currency Management Market worth $1,305.97 billion by 2030, growing at a CAGR of …
The "Currency Management Market by Offering (Services, Software), Type (Fixed Currency Exchange, Floating Currency Exchange), End-User - Global Forecast 2024-2030" report has been added to 360iResearch.com's offering.
Request a Free Sample Report @ https://www.360iresearch.com/library/intelligence/currency-management
Currency management encompasses strategies, methodologies, and tools employed by firms and financial institutions to address risks and potential benefits associated with currency fluctuations, involving activities such as hedging, arbitrage, and strategic investment planning to mitigate negative financial impacts…
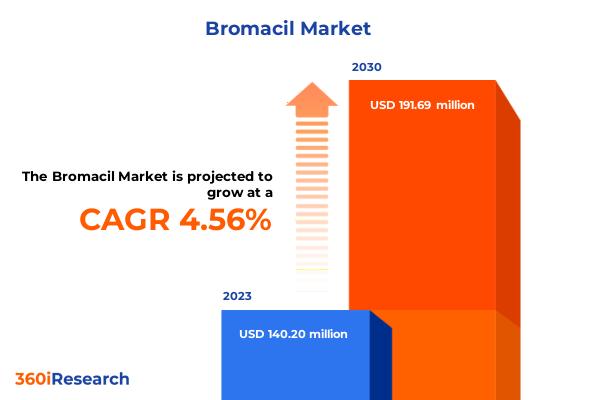
Bromacil Market worth $191.69 million by 2030, growing at a CAGR of 4.56% - Excl …
The "Bromacil Market by Formulation (Granular Formulation, Liquid Formulation), Distribution Channel (Offline, Online) - Global Forecast 2024-2030" report has been added to 360iResearch.com's offering.
Request a Free Sample Report @ https://www.360iresearch.com/library/intelligence/bromacil
Bromacil, a herbicide used to control broadleaf weeds and grasses, is vital for vegetation management in agricultural and non-cropland areas due to its soil-residual activity and efficacy in preventing weed growth, ultimately enhancing agricultural productivity and land utility. Applications span agricultural…
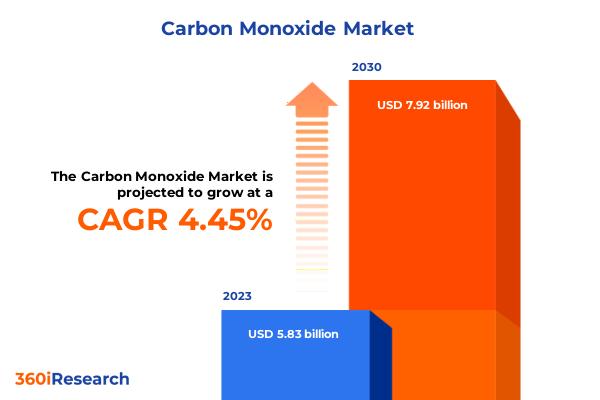
Carbon Monoxide Market worth $7.92 billion by 2030, growing at a CAGR of 4.45% - …
The "Carbon Monoxide Market by Purity Level (98% - 99%, Over 99%), Application (Chemical Synthesis, Food & Beverage, Fuel) - Global Forecast 2024-2030" report has been added to 360iResearch.com's offering.
Request a Free Sample Report @ https://www.360iresearch.com/library/intelligence/carbon-monoxide
The scope of carbon monoxide (CO) market research encompasses comprehensive evaluations of production, distribution, application, and end-use industries, providing insights into current market size, projected growth, and future demand across key regions such as North…
More Releases for Drug
Alcohol Testing And Drug Testing Equipment Market 2025 Segmentation, Application …
Market Study Report, LLC, has compiled an exhaustive research study of the ‘Alcohol Testing And Drug Testing Equipment market’, detailing every single market driver and intricately analyzing the business vertical. This ‘Alcohol Testing And Drug Testing Equipment market’ study will aid in seeking out new business opportunities and fine-tuning existing marketing strategies through insights regarding SWOT analysis, market valuation, competitive spectrum, regional share, and revenue predictions.
Alcohol abuse and drug…
How much Diabetes Drug Market Impact Worldwide Medical Drug Industry?
Diabetes Drug Market From an insight perspective, the market report focuses on various levels of analyses — industry analysis, market rank analysis, and company profiles, which together comprise and discuss basic views on the competitive landscape, high-growth regions, and countries as well as their respective regulatory policies, Types ,Applications and opportunities in the market.
Diabetes is a metabolic disorder in which the body glucose level is elevated. There are two types of diabetes…
Hepatitis Drug Market Hepatitis Drug Clinical Pipeline Report 2023
For Report Sample Contact: neeraj@kuickresearch.com or +91-11-47067990
Report Table of Contents
1. Introduction to Hepatitis Disease
1.1 Prologue
1.1.1 History of Hepatitis
1.1.2 Causes of Hepatitis Disease
1.2 Types of Viruses which are Responsible for Hepatitis Disease
2. Global Prevalence of Hepatitis Infection
3. Available Drug Classes for Hepatitis Disease Treatment
3.1 Interferon Alfa Therapy
3.2 Protease Inhibitors Therapy
3.3 Polymerase…
Microcapsules Drug Delivery Market Microcapsules Drug Delivery Sales Report 2022
For Report Sample Contact: neeraj@kuickresearch.com or +91-11-47067990
Report Table of Contents
1. Indispensable Advent of Microcapsules
1.1 Trajectory of Microencapsulation
1.2 Why and wherefores for Microencapsulation
2. Characterization of Microcapsules
2.1 Composition of Microcapsules
2.2 Parameters Influencing Microcapsules
3. Engineering Technology of Microcapsules
3.1 Physical Manufacturing Technologies
3.2 Physicochemical and Chemical Technologies
4. Applicability of Microcapsules
4.1 Microcapsules in Pharmaceuticals
4.2 Microcapsules in Nutraceuticals
…
AstraZeneca’s NMOSD Drug receives orphan drug designation
April 18, 2017 Orphan Drug
European Medicine Agency has Announced orphan drug designation for inebilizumab (earlier known as MEDI-551) developed by AstraZeneca PLC (AZN) for the treatment of neuromyelitis optica spectrum disorder (NMOSD). The drug has already received orphan drug status from US Food and Drug Administration (FDA). NMOSD, also called Devic’s disease, is a rare, autoimmune disorder of the central nervous system (CNS) that affects the functioning…
Drug Discovery Strategies, PBPK Modeling and Drug-Drug Interaction Explored and …
This year’s 12th annual ADMET conference and exhibition promises to deliver appearances from experienced pharmaceutical professionals from ADME and PK/PD organisations such as AbbVie, GSK, Roche and Bayer when it returns to London this June.
The carefully selected speaker line up will be addressing early ADME application strategies and discussing the latest technologically advanced screening and testing models along with exploring ADMET technologies which will identify properties of a drug…