Press release
Arrival of Large Bore Venous Transcatheter Therapies Amplifying the Need for Efficient Closure
The current influx of regulatory approvals for transcatheter interventions requiring large bore venous access is highlighting the absence of quick efficient vascular perforation closure. Current options for closure are either tedious and lengthy manual compression, or off-label workarounds with multiple small-bore closure devices. Venock's dedicated large bore closure device will respond to this unmet need in the market by providing immediate and efficient closure of large bore venous access sites, enhancing patient comfort while providing significant cost savings and efficiency to the cathlab. Regulatory approval of the device is forthcoming.The recent US and EU regulatory approvals of leadless pacemakers (Medtronic's Micra and Abbott's Avier), as well as the tricuspid valve replacement system (Edwards's EVOQUE), and most recently the tricuspid valve repair system (Abbott's Triclip) are an early indication of the influx of large bore venous transcatheter therapies coming to market.
The only way to close these access sites is either manual compression (sometimes with a "Figure of 8" stitch), or off-label workarounds with multiple closure devices which are indicated for small bore sites. Dr Richard R. Heuser, a widely renowned cardiologist and professor of Medicine of the University of Arizona affirms, "Venous procedures using large bore catheters are becoming more and more common, particularly as we work on tricuspid valves and other structural heart problems. It's been my experience, that the only devices out there are currently used off-label and are very inconvenient. You have to prepare closure before the procedure and it sometimes requires multiple different closure devices, which makes the procedure expensive."
The archaic process of manual compression is time consuming (requiring hours of recovery time), thus expensive, and very burdensome for the patient. This process slows down discharge and patient turnover, negatively affecting the number of therapies which can be done per day and thus overall efficiency. Additionally, the use of multiple small-bore devices not only increases procedural time and cost, but also adds unnecessary complexity to the closure procedure.
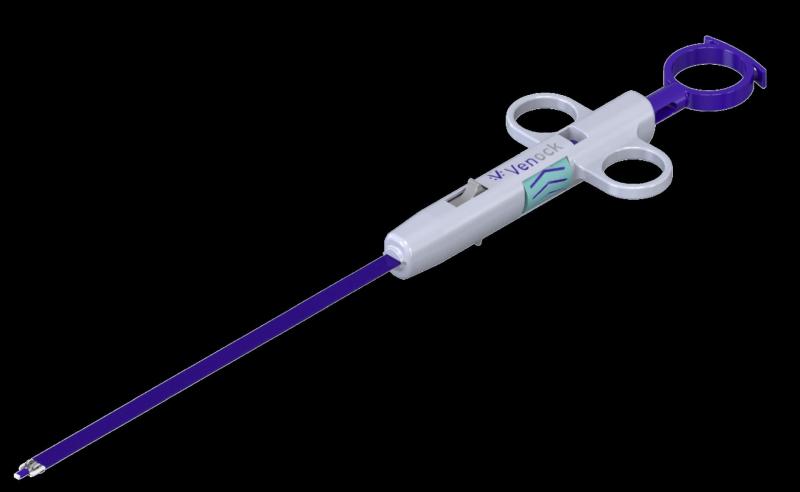
Venock's dedicated large bore closure device (Figure 1) will respond to this unmet need in the market by providing quick efficient closure in 1 minute, removing the need for the 6 to 8 hours of bedrest, enhancing patient comfort while providing significant cost savings and efficiency to the cathlab.
Dr Richard R. Heuser agrees: "Venock is a one stop shopping device that is cost-effective and importantly will reduce time in the outpatient center or the hospital with cost savings for Medicare, health plans as well as the hospital and outpatient center."
More:
www.venock.com
www.venock.com/index.php/whitepaper.html
CAUTION: The Venock system is not approved for sale or investigational use.
VENOCK INC.
Terry Barnes, CEO
521 Fifth Avenue, Floor 17
New York, NY 10175 - USA
Email: terry.barnes@venock.com
Mobile: +49 170 557 0073
www.venock.com
Venock Inc. is a privately held US medical device company headquartered in New York City, with the subsidiary Venock Medical GmbH in Munich, Germany. Its Vascular Closure Device is designed to close very large perforations that can be as big as the diameter of the vein or the artery.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Arrival of Large Bore Venous Transcatheter Therapies Amplifying the Need for Efficient Closure here
News-ID: 3492760 • Views: …
More Releases from Venock Inc.
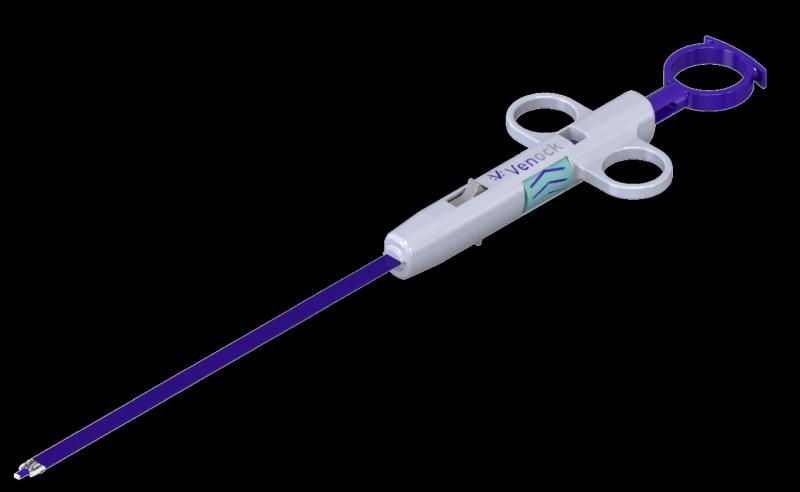
Economic Study Shows Significant Cost Savings when Using Venock Device for Large …
US-based Venock Inc. has developed a vascular closure device which will rapidly close large bore venous access sites following transcatheter procedures. According to a new study, this device could provide cost savings of more than $1,700 per procedure. In addition, the device will enable same-day patient discharge providing a cost savings of up to $6,290 per patient. The Venock device replaces the tedious and lengthy manual compression steps currently used…
More Releases for Abbott
Global Rapid Diagnostics Market 2022 Industry Challenges by Players - Roche Diag …
The new MarketQuest.biz exploration report principally focuses on the development speed of the Global Rapid Diagnostics Market from 2022-2028, identifying the foremost necessary market participants. To form an announcement regarding the extended run growth opportunities of the market Rapid Diagnostics, a comprehensive investigation is a dole out along with a singular investigation technique log.
The analysis method consists of primary and secondary information to extend the dependableness and accuracy of the…
Arteriotomy Closure Devices Market 2022 Precise Outlook - Abbott, Abbott, Cardin …
The "Arteriotomy Closure Devices" Market report offers qualitative and quantitative insights and a detailed analysis of market size & growth rate for all possible segments in the market. The Global Arteriotomy Closure Devices Industry presents a market overview, product details, classification, and market concentration. The report also provides an in-depth survey of key players in the market which is based on various competitive intelligence parameters like company profiles, product picture…
Home Monitoring Equipment Market Report- Growth Rate by Application | Abbott, GE …
ReportsnReports added Home Monitoring Equipment Market Research Report created by Report Consultant, which offers detailed insights, revenue details, and other information regarding the global market, and the various trends, drivers, restraints, opportunities, and market till 2028. Home Monitoring Equipment Market Report offers detailed information regarding the leading key players operating in the market, their financials, supply chain trends, technological innovations, key developments, apart from future strategies, acquisitions and mergers, and…
Global LIMS Market 2020 Business Strategies – Siemens SA, Labware, Star LIMS ( …
The market report titled “LIMS Market: Global Industry Analysis, Size, Share, Growth, Trends, and Forecasts 2016–2024” and published by Zion Market Research will put forth a systematizedevaluation of the vital facets of the global LIMS market. The report willfunction as a medium for the better assessment of the existing and future situations of the global market. It will be offering a 360-degree framework of the competitive landscape and dynamics of…
Nutrition and Supplements Market – Recent Activity Focus on Nestle, Bayer, Amw …
"Nutrition and Supplements Market research report delivers a close watch on leading competitors with strategic analysis, micro and macro market trend and scenarios, pricing analysis and a holistic overview of the market situations in the forecast period.
Get Exclusive FREE Sample Copy Of this Report @ https://www.upmarketresearch.com/home/requested_sample/24594
UpMarketResearch offers a latest published report on “Global Nutrition and Supplements Market Analysis and Forecast 2019 - 2026” delivering key insights and providing a competitive…
Nutrition and Supplements Market Professional Survey Report 2018 | Nestle, Bayer …
Researchmoz added Most up-to-date research on "Global Nutrition and Supplements Market Professional Survey Report 2018" to its huge collection of research reports.
This report studies the global Nutrition and Supplements market status and forecast, categorizes the global Nutrition and Supplements market size (value & volume) by manufacturers, type, application, and region. This report focuses on the top manufacturers in North America, Europe, Japan, China, India, Southeast Asia and other regions (Central…