Press release
Regulatory Affairs Outsourcing Market Size is Anticipated to Boost at a CAGR of 19 % by 2028 | Transparency Market Research
Regulatory Affairs Outsourcing offers companies several benefits, including access to specialized expertise, cost savings, flexibility, and scalability. By outsourcing regulatory functions to external service providers, companies can leverage the knowledge and experience of regulatory professionals without the need to maintain an in-house regulatory affairs team. This allows companies to focus on their core competencies, accelerate product development timelines, and navigate regulatory challenges more efficiently.Regulatory Affairs Outsourcing market is estimated to attain a valuation of US$ 17.3 Bn by the end of 2028, states a study by Transparency Market Research (TMR). Besides, the report notes that the market is prognosticated to expand at a CAGR of 19% during the forecast period, 2021-2028
Get a Sample Copy of the Regulatory Affairs Outsourcing Market Research Report -https://www.transparencymarketresearch.com/sample/sample.php?flag=S&rep_id=3528&utm_source=openpr_amitugare&utm_medium=openpr
The significant players operating in the global Regulatory Affairs Outsourcing market are
Accell Clinical Research, LLC, Charles River Laboratories International, Inc., Clinilabs, Inc., Covance, Inc., Criterium, Inc., ICON plc, Medpace, Inc., PAREXEL International Corporation, Pharmaceutical Product Development, (PPD) LLC, Promedica International, Quintiles Transnational Corporation, and WuXi App Tec.
Key Drivers of Regulatory Affairs Outsourcing:
Complex Regulatory Landscape: The global regulatory environment for healthcare products is complex and constantly evolving, with multiple regulatory agencies, guidelines, and requirements that vary by region and product type. Outsourcing regulatory affairs allows companies to access specialized knowledge and keep pace with regulatory changes without the need for extensive internal resources.
Cost Containment: Outsourcing regulatory affairs can be cost-effective compared to maintaining an in-house regulatory team, particularly for small and medium-sized companies with limited resources. External service providers often offer flexible pricing models, allowing companies to scale regulatory activities according to their needs and budgets.
Access to Expertise: Regulatory affairs outsourcing provides access to regulatory experts with specialized knowledge and experience in specific therapeutic areas, product types, or regions. These experts can provide strategic guidance, regulatory intelligence, and practical insights to navigate complex regulatory pathways and optimize regulatory outcomes.
Time Efficiency: Outsourcing regulatory functions can accelerate product development timelines by streamlining regulatory processes, reducing administrative burdens, and facilitating timely submissions and approvals. External service providers can dedicate resources to regulatory activities, allowing internal teams to focus on other priorities.
Risk Mitigation: Regulatory affairs outsourcing can help companies mitigate regulatory risks by ensuring compliance with applicable laws, regulations, and quality standards. External service providers can conduct regulatory assessments, gap analyses, and compliance audits to identify potential issues and implement corrective actions proactively.
Recent Developments in Regulatory Affairs Outsourcing:
Increased adoption of outsourcing models by pharmaceutical, biotechnology, medical device, and diagnostics companies to streamline regulatory operations, optimize resource allocation, and enhance regulatory compliance.
Expansion of regulatory affairs outsourcing services to include specialized areas such as pharmacovigilance, medical writing, regulatory intelligence, market access, and regulatory technology (RegTech) solutions.
Growth of the regulatory affairs outsourcing market globally, driven by the globalization of clinical trials, the harmonization of regulatory requirements, and the increasing complexity of product development pathways.
Integration of digital technologies, data analytics, and automation tools into regulatory affairs outsourcing services to improve efficiency, transparency, and collaboration between companies and external service providers.
Collaboration between regulatory affairs outsourcing providers, industry associations, regulatory agencies, and academia to address emerging regulatory challenges, promote best practices, and advance regulatory science and innovation.
Buy this Premium Research Report: - https://www.transparencymarketresearch.com/checkout.php?rep_id=3528<ype=S
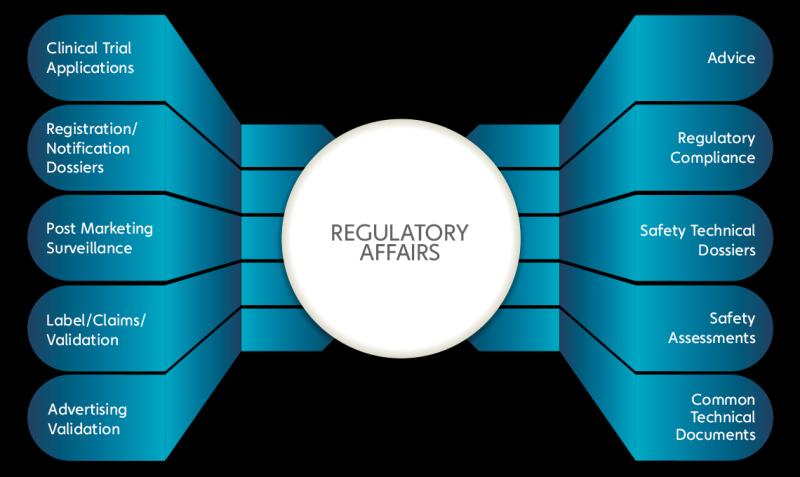
Market Segmentation -
Service
Regulatory Affairs
Clinical Trial Applications and Product Registrations
Regulatory Writing and Publishing
Regulatory Consulting and Legal Representation
Others
This Report lets you identify the opportunities in Regulatory Affairs Outsourcing Market by means of a region:
North America (the United States, Canada, and Mexico)
Europe (Germany, UK, France, Italy, Russia, Turkey, etc.)
Asia-Pacific (China, Japan, Korea, India, Australia, and Southeast Asia (Indonesia, Thailand, Philippines, Malaysia, and Vietnam))
South America (Brazil etc.) The Middle East and Africa (North Africa and GCC Countries)
Key Features of the Regulatory Affairs Outsourcing Market Report: -
➤ Analyze competitive developments such as expansions, deployments, new product launches, and market acquisitions.
➤ Examine the market opportunities for stakeholders by identifying higher growth sections.
➤ To study and analyze the global Regulatory Affairs Outsourcing industry status and forecast including key regions.
➤ An in-depth analysis of key product segments and application spectrum, providing strategic recommendations to incumbents and new entrants to give them a competitive advantage over others.
➤ It provides a comprehensive analysis of key regions of the industry as well as a SWOT analysis and Porter's Five Forces analysis to provide a deeper understanding of the market.
➤ It helps you make strategic business decisions and investment plans.
More Trending Reports by Transparency Market Research -
Medical Membrane Market: https://www.globenewswire.com/en/news-release/2024/03/07/2842005/32656/en/Medical-Membrane-Market-Size-Share-to-Surpass-USD-10-2-billion-by-2031-Analysis-by-Transparency-Market-Research-Inc.html
Stem Cell Manufacturing Market: https://www.globenewswire.com/en/news-release/2024/03/07/2842010/32656/en/Stem-Cell-Manufacturing-Market-Worth-USD-26-6-billion-Growing-At-9-2-CAGR-by-2033-Report-By-Transparency-Market-Research-Inc.html
About Us Transparency Market Research
Transparency Market Research, a global market research company registered at Wilmington, Delaware, United States, provides custom research and consulting services. The firm scrutinizes factors shaping the dynamics of demand in various markets. The insights and perspectives on the markets evaluate opportunities in various segments. The opportunities in the segments based on source, application, demographics, sales channel, and end-use are analysed, which will determine growth in the markets over the next decade.
Our exclusive blend of quantitative forecasting and trends analysis provides forward-looking insights for thousands of decision-makers, made possible by experienced teams of Analysts, Researchers, and Consultants. The proprietary data sources and various tools & techniques we use always reflect the latest trends and information. With a broad research and analysis capability, Transparency Market Research employs rigorous primary and secondary research techniques in all of its business reports.
𝐂𝐨𝐧𝐭𝐚𝐜𝐭 𝐔𝐬
Transparency Market Research Inc.
CORPORATE HEADQUARTER DOWNTOWN,
1000 N. West Street,
Suite 1200, Wilmington, Delaware 19801 USA
Tel: +1-518-618-1030
USA - Canada Toll Free: 866-552-3453
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Regulatory Affairs Outsourcing Market Size is Anticipated to Boost at a CAGR of 19 % by 2028 | Transparency Market Research here
News-ID: 3431405 • Views: …
More Releases from Transparency Market Research

Handheld Marijuana Vaporizer Market Key Drivers, Market Research, and Insights f …
The global handheld marijuana vaporizer market has witnessed remarkable growth in recent years, attributed to the increasing acceptance of medicinal marijuana and the demand for convenient and discreet consumption methods. Valued at US$ 5 billion in 2021, the market is projected to surge at a CAGR of 13.4% to reach US$ 15.9 billion by 2031. This article delves into the factors driving this growth, the evolving market landscape, and the…
Disinfectant Wipes Market Forecast 2020-2030 - Market Size, Drivers, Trends, And …
The COVID-19 pandemic has precipitated a remarkable surge in the demand for disinfectant products, with a notable emphasis on disinfectant wipes as an essential tool in maintaining hygiene and preventing the spread of pathogens. This research report delves into the multifaceted landscape of the disinfectant wipes market, elucidating key trends, drivers, and innovations shaping the industry.
𝐆𝐞𝐭 𝐒𝐚𝐦𝐩𝐥𝐞 𝐏𝐃𝐅 𝐂𝐨𝐩𝐲 𝐨𝐟 𝐭𝐡𝐢𝐬 𝐫𝐞𝐬𝐞𝐚𝐫𝐜𝐡 𝐫𝐞𝐩𝐨𝐫𝐭 @ https://www.transparencymarketresearch.com/sample/sample.php?flag=S&rep_id=70359
Key Players and Market Developments
Key players…
Tissue Banking Market is Expected to Reach US$ 2,903.3 Million to 2026| TMR Stud …
The global 𝐭𝐢𝐬𝐬𝐮𝐞 𝐛𝐚𝐧𝐤𝐢𝐧𝐠 𝐦𝐚𝐫𝐤𝐞𝐭 reached a value of US$ 1,056.4 million in 2017 and is projected to nearly triple to US$ 2,903.3 million by 2026, with a robust Compound Annual Growth Rate (CAGR) of approximately 12.0% from 2018 to 2026. Factors such as increasing awareness about tissue donation, technological advancements, and a growing target patient population are anticipated to propel market growth during this period.
Moreover, the market is expected…
Vascular Closure Devices Market to reach US$ 1 Billion by 2027: TMR Study
This report by Transparency Market Research, Inc. assesses the present state and future growth potential of the global 𝐯𝐚𝐬𝐜𝐮𝐥𝐚𝐫 𝐜𝐥𝐨𝐬𝐮𝐫𝐞 𝐝𝐞𝐯𝐢𝐜𝐞𝐬 𝐦𝐚𝐫𝐤𝐞𝐭. It features a comprehensive executive summary, offering insights into various segments of the market. Additionally, the report provides detailed analysis and data on product, access type, application, end user, and regional segments within the global market.
Vascular closure devices is estimated to reach a value of ~US$ 1 Bn…
More Releases for Regulatory
Complex Regulatory Frameworks
It is challenging for new entrants to enter the FinTech industry because of its complex regulatory framework. All FinTech companies must comply with compliance requirements even before they begin operations, which increases their costs and creates a significant barrier for startups. While regulations are needed to protect consumers, a number of existing laws are slowing down the growth of many Indian FinTech companies, thereby extending their time to reach the…
Healthcare Contract Research Outsourcing Market: Complex Regulatory and Growing …
The global healthcare contract research outsourcing market was valued at US$ 30.0 Bn in 2016 and is projected to expand at a cumulative annual growth rate (CAGR) of 6.8% from 2017 to 2025 according to a new report published by Transparency Market Research (TMR) titled “Healthcare Contract Research Outsourcing Market Global Industry Analysis, Size, Share, Growth, Trends, and Forecast, 2017–2025” the report suggests that increase product pipeline and efforts for…
Monetum Gains Swiss Regulatory Approval
The Swiss-based Cryptocurrency Exchange Joins Financial Services Standards Association (VQF)
[ZUG, SWITZERLAND, 24th FEBRUARY 2021] - Zug-headquartered company Fenice Holding SA (Monetum) is now a member of the Swiss Financial Services Standards Association (VQF). VQF itself is the largest cross-industry Self-Regulatory Organization (SRO) with the longest history that meets the standards established by the Swiss Financial Market Supervisory Authority (FINMA).
Officially authorised by FINMA, VQF is entrusted to oversee the compliance…
Regulatory Reporting Solutions Market 2019| Helps To Improve Accuracy, Quality A …
"Regulatory Reporting Solutions Market: Global Industry Analysis 2013-2017 and Opportunity Assessment2018-2028" is the latest addition to MarketResearchReports.Biz industry research reports collection.
Regulatory reporting solutions is a purposely build solution which is adopted by enterprises to automate workflow process for shareholding disclosure monitoring and reporting. In enterprise application, the need of regulatory systems is growing rapidly in order to manage the increasing enforcement actions. It also helps to improve accuracy, quality, and…
South Africa Upstream Fiscal and Regulatory Report 2017 - Pending Legislation Cr …
Presented report, South Africa Upstream Fiscal and Regulatory Report 2017 - Pending Legislation Creates Regulatory Uncertainty, presents the essential information relating to the terms which govern investment into South Africa’s upstream oil and gas sector. The report sets out in detail the contractual framework under which firms must operate in the industry, clearly defining factors affecting profitability and quantifying the state’s take from hydrocarbon production. Considering political, economic and industry…
Regulatory Affairs Outsourcing Market (Services - Regulatory Submissions, Clinic …
This research study analyzes the market for regulatory affairs outsourcing services in terms of revenue (US$ Mn). The stakeholders of this report comprises the clinical research organizations. The global regulatory affairs outsourcing market has been broadly segmented on the basis of services (Regulatory Submissions, Clinical Trial Applications and Product Registrations, Regulatory Writing and Publishing, Regulatory Consulting and Legal Representation and others regulatory affairs, and Geography (North America, Europe, Asia Pacific,…