Press release
Viral and Non-Viral Vector Manufacturing Market Size, Share and Scope Analysis to 2030
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the " "Global Viral and Non-Viral Vector Manufacturing Market (Vector Type (Viral Vector (Adenoviral Vector, Retroviral Vector, Adeno-Associated Viral Vector, Lentiviral Vector, Vaccinia Viral Vector, Other Viral Vector), Non-Viral Vectors, (Plasmid DNA, Lipid-Based Non-Viral Vector, Polymer-Based Non-Viral Vector, Other Non-Viral Vector (Peptide-Based And Hybrid/Combination))), Diseases (Cancer, Genetic Disorder, And Infectious Diseases), Application (Gene Therapy (Viral Vector, Non-Viral Vector), Vaccinology (Viral Vector, Non-Viral Vector), Cell Therapy (Viral Vector, Non-Viral Vector) And Other Applications (Viral Vector, Non-Viral Vector))) - Trends, Industry Competition Analysis, Revenue and Forecast To 2030."Get Free Sample Copy of Report :https://www.insightaceanalytic.com/request-sample/1263
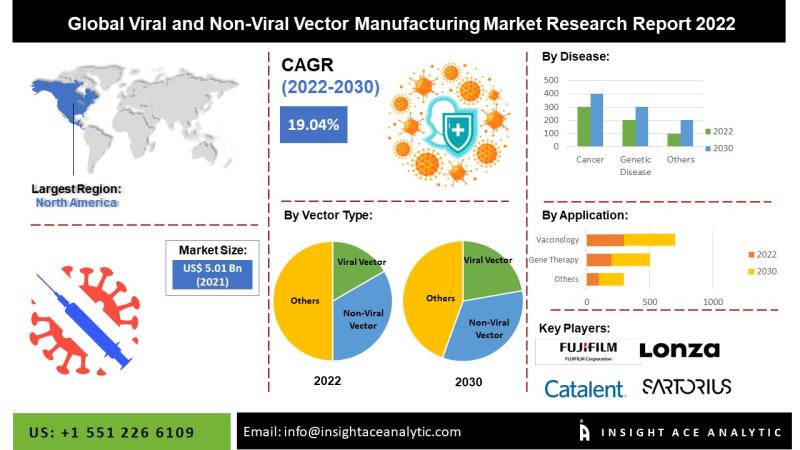
According to the latest research by InsightAce Analytic, the global viral and non-viral vector manufacturing market is valued at US$ 5.01 billion in 2021, and it is expected to reach US$ 23.49 billion by 2030, with a CAGR of 19.04% during the forecast period of 2022-2030.
Advanced gene and cell therapies, with the use of vectors, have significantly impacted the field of medicine. Therapies requiring genetic modification have demonstrated tremendous potential for treating several chronic diseases and neurological disorders. Gene therapy has significant potential for treating and managing a variety of disorders. Due to their exceptional capacity to transport replicas of therapeutic genes to host cells, viral vectors are the most often employed agents in gene therapy. Adeno-associated vectors (AAV) are the most promising among this class of vectors due to their transitory nature, ability to transduce dividing and non-dividing cells, and low immunogenic disposition. The promising results of vector-based therapies in several clinical studies have further emphasized the development of various viral and non-viral vectors to address unmet medical needs.
Multiple factors, including the rising prevalence of fatal diseases and the increasing usage of next-generation technologies in developing innovative medicines, are the primary drivers of the market's expansion. The increasing prevalence of vector-based gene and cell treatments, the high demand for customised medications, the rising R&D spending for vector-based therapeutic breakthroughs, and the well-established healthcare infrastructures are anticipated to drive considerable market expansion in the coming years. Increasing incidences of chronic and infectious illnesses are increasing the need for therapies requiring a genetic alteration, consequently presenting several chances for the growth of the viral and non-viral vector manufacturing market.
On the other side, the high cost of vector manufacturing, complications associated with large-scale production of viral and non-viral vectors, and low transfection efficiency may hinder the market development in the coming years.
North America is projected to lead the global viral and non-viral vector manufacturing market in the coming years (2020-2030), owing to the increasing R&D activities in vector-based therapy innovations and the rapid adoption of advanced therapy techniques.
Key market players operating in the viral and non-viral vector manufacturing market include Boehringer Ingelheim (Germany), Catalent, Inc. (US), FUJIFILM Holdings Corporation(Japan), Danaher Corporation (US), Genscript Biotech Corporation (US), Lonza Group AG (Switzerland), Merck KGaA Inc. (Germany), Oxford Biomedica plc (UK), Sartorius AG (Germany), Takara Bio Inc. (Japan), Thermo Fisher Scientific Inc. (US), Wuxi AppTec (China), Acuitas Therapeutics (Canada), Evonik Industries AG (Germany), Exelead, Inc. (US), Entos Pharmaceuticals (Canada), Genevant Sciences GmbH (Switzerland), T&T Scientific Corporation (US), Moderna, Inc. (US), CureVac N.V. (Germany), Cognate BioServices Inc. (Cobra Biologics), Genezen Laboratories, Yposkesi, Waisman Biomanufacturing, Advanced Bioscience Laboratories, Inc. (Abl Inc.), Novasep Holding S.A.S., Batavia Biosciences B.V., Biovion Oy, Sirion Biotech Gmbh., Virovek Incorporation, Biontech Imfs Gmbh, Vivebiotech S.L., Creative Biogene, Uniqure NV, Cell and Gene Therapy Catapult, Cevec. Pharmaceuticals Gmbh among others.
Key developments in the market:
In May 2022, Catalent launched its latest UpTempo VirtuosoTM platform method for developing and CGMP production of adeno-associated viral (AAV) vectors. The UpTempo Virtuoso platform standardizes and streamlines several time-consuming procedures in AAV manufacture to drastically minimize the time from gene to clinic and enable quick clinical assessment of the first patient.
In December 2021, GenScript ProBio (US) opened China's most prominent commercial GMP plasmid manufacturing facility. The 6,400-square-meter manufacturing plant enables the company to offer global customers a one-stop service for plasmids - from the preclinical study (IIT) to the investigational new drug (IND) filing to clinical trial and commercial manufacturing - to accelerate the innovation and development of high-quality cell and gene therapy mRNA drugs.
In December 2021, Oxford Biomedica plc (UK) extended a commercial supply agreement with Novartis to manufacture lentiviral vectors for several Novartis CAR-T products.
In October 2021, Matica Biotechnology, Inc, a (CDMO) specializing in the clinical and commercial production of cell and gene therapies, announced a joint research agreement (JRA) with Sartorius, a leading international partner of the biopharmaceutical industry. Under this agreement, Matica Bio and Sartorius will work on several studies to streamline and optimize PAT technologies, automation software, and single-use platforms offered by Sartorius for large-scale vector production.
In April 2021, Oxford Biomedica plc (UK) signed a new three-year Development & Supply Agreement ("DSA") with Boehringer Ingelheim to manufacture & supply various types of the viral vector.
In Jan 2021, Thermo Fisher Scientific Inc. and Groupe Novasep SAS (Novasep) announced that Thermo Fisher acquired Novasep's viral vector manufacturing company in Belgium, Henogen S.A. The viral vector manufacturing division of Novasep offers contract manufacturing services for vaccines and medicines to biotechnology and major biopharma clients.
Enquiry Before Buying @ https://www.insightaceanalytic.com/enquiry-before-buying/1263
Market Segments
Global Viral and Non-Viral Vector Manufacturing Market, by Vector Type, 2020-2030 (Value US$ Mn)
Viral Vector
oAdenoviral Vector
oRetroviral Vector
oAdeno-Associated Viral Vector
oLentiviral Vector
oVaccinia Viral Vector
oOther Viral Vector
Non-Viral Vectors
oPlasmid DNA
oLipid-Based Non-Viral Vector
oPolymer-Based Non-Viral Vector
oOther Non-Viral Vector (Peptide-Based and Hybrid/Combination)
Global Viral and Non-Viral Vector Manufacturing Market, by Disease, 2020-2030 (Value US$ Mn)
Cancer
Genetic Disease
Infectious Disease
Cardiovascular Disease
Other Diseases
Global Viral and Non-Viral Vector Manufacturing Market, by Application, 2020-2030 (Value US$ Mn)
Gene Therapy
oViral Vector
oNon-Viral Vector
Vaccinology
oViral Vector
oNon-Viral Vector
Cell Therapy
oViral Vector
oNon-Viral Vector
Other Applications
oViral Vector
oNon-Viral Vector
Global Viral and Non-Viral Vector Manufacturing Market, by Region, 2020-2030 (Value US$ Mn)
North America
Europe
Asia Pacific
Latin America
Middle East & Africa
North America Viral and Non-Viral Vector Manufacturing Market, by Country, 2020-2030 (Value US$ Mn)
U.S.
Canada
Europe Viral and Non-Viral Vector Manufacturing Market, by Country, 2020-2030 (Value US$ Mn)
Germany
France
Italy
Spain
Russia
Rest of Europe
Asia Pacific Viral and Non-Viral Vector Manufacturing Market, by Country, 2020-2030 (Value US$ Mn)
India
China
Japan
South Korea
Australia & New Zealand
Latin America Viral and Non-Viral Vector Manufacturing Market, by Country, 2020-2030 (Value US$ Mn)
Brazil
Mexico
Rest of Latin America
Middle East & Africa Viral and Non-Viral Vector Manufacturing Market, by Country, 2020-2030 (Value US$ Mn)
GCC Countries
South Africa
Rest of Middle East & Africa
For Customised Information @ https://www.insightaceanalytic.com/customisation/1263
Other Related Reports Published by InsightAce Analytic:
Global Gene Therapy for Blood Disorders Market
Global Gene Therapy For Retinal Diseases Market
Global Cell and Gene Therapy Drug Delivery Devices Market
Global iPSCs Manufacturing Services Market
Global mRNA Synthesis and Manufacturing Services Market
Why should buy this report:
To receive a comprehensive analysis of the prospects for the global viral and non-viral vector manufacturing market
To receive an industry overview and future trends of the viral and non-viral vector manufacturing market
To analyze the viral and non-viral vector manufacturing market drivers and challenges
To get information on the viral and non-viral vector manufacturing market size (Value US$ Mn) forecast to 2030
Significant investments, mergers & acquisitions in the viral and non-viral vector
About Us:
InsightAce Analytic is a market research and consulting firm that enables clients to make strategic decisions. Our qualitative and quantitative market intelligence solutions inform the need for market and competitive intelligence to expand businesses. We help clients gain a competitive advantage by identifying untapped markets, exploring new and competing technologies, segmenting potential markets, and repositioning products. Our expertise is in providing syndicated and custom market intelligence reports with an in-depth analysis with key market insights in a timely and cost-effective manner.
Contact Us:
InsightAce Analytic Pvt. Ltd.
Tel.: +1 718 593 4405
Email: info@insightaceanalytic.com
Site Visit: www.insightaceanalytic.com
Follow Us on LinkedIn @ bit.ly/2tBXsgS
Follow Us On Facebook @ bit.ly/2H9jnDZ
Corporate Office :
Office No.3050, 3rd Floor Marvel Fuego, Magarpatta Rd, Hadapsar, Pune, Maharashtra 411028
Sales Office (U.S.) :
344 Grove St Unit #967 Jersey City, NJ 07302
info@insightaceanalytic.com
info@insightaceanalytic.com
North America:
+1 551 226 6109
Asia:
+91 79 72967118
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Viral and Non-Viral Vector Manufacturing Market Size, Share and Scope Analysis to 2030 here
News-ID: 2987473 • Views: …
More Releases from Insightace Analytics
Plastic Recycling Market Deep Research Report with Forecast to 2031
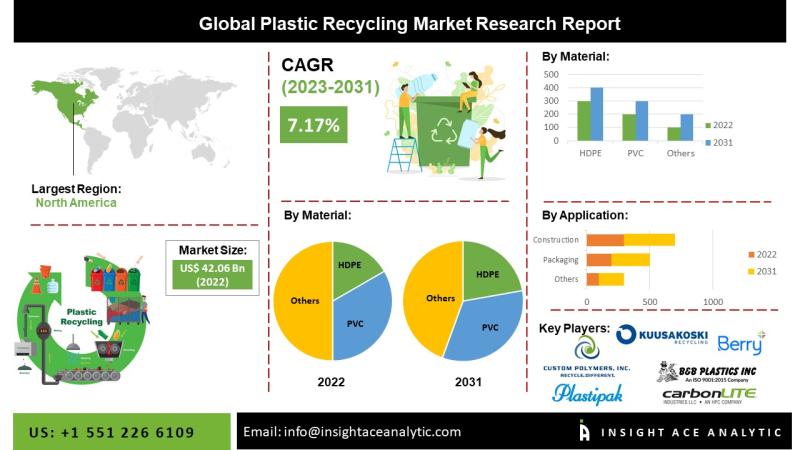
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Plastic Recycling Market Size, Share & Trends Analysis Report By Material (PET, PP, HDPE, LDPE, PS, PVC), Application (Packaging, Automotive, Construction, Textiles) - Market Outlook And Industry Analysis 2031"
The Global Plastic Recycling Market Size is valued at 42.06 billion in 2022 and is predicted to reach 77.19 billion by the year 2031 at a 7.17%…
The Lipid Nanoparticles (LNPs) CDMO Market Size, Share and Trends Analysis to 20 …
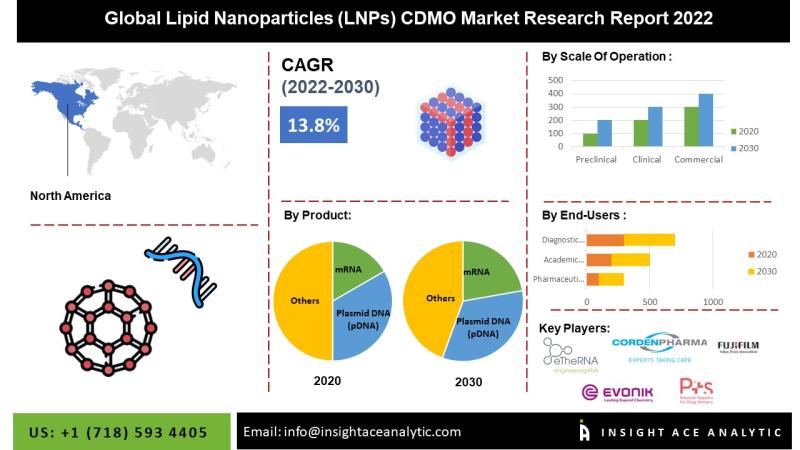
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the " "Global Lipid Nanoparticles (LNPs) CDMO Market Focus on Nucleic Acids LNPs Segmented By Product (mRNA , Plasmid DNA (pDNA), siRNA, saRNA, microRNA, and Others), Scale Of Operation (Preclinical, Clinical , and Commercial), End-Users (Pharmaceutical Companies, Academic Research Institute, and Diagnostic Laboratories)- Trends, Industry Competition Analysis, Revenue and Forecast To 2030."
According to the latest research by…
Green Hydrogen Market Size, Share and Trends Analysis Report 2023-2030
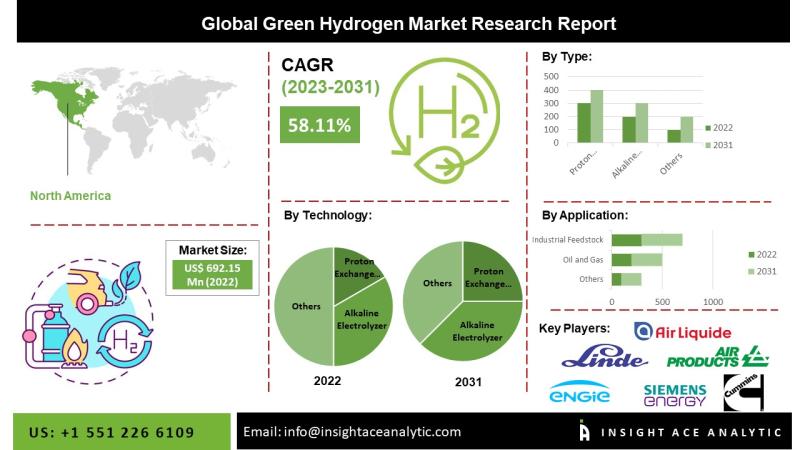
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Green Hydrogen Market Size, Share & Trends Analysis Report by Application (Oil and Gas, Industrial Feedstock, Mobility, Power Generation) And Technology (Proton Exchange Membrane Electrolyzer, Alkaline Electrolyzer, Anion Exchange Membrane, And Solid Oxide Electrolyzer)- Market Outlook and Industry Analysis 2031"
According to company's newest research, the global green hydrogen market size was valued at US$…
Vaccine Cold Chain Logistics Market Detailed Study Analysis and Forecast to 2030
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Vaccine Cold Chain Logistics Market By Services (Storage, Packaging, And Transportation), By Packaging Methods (Dry Ice, Liquid Nitrogen, Gel Packs), Mode Of Transportation (Ground, Air, Ocean), Holding Temperature Range (Vaccines Requiring Refrigerated Temperature, Vaccines Requiring Frozen Temperature, Vaccines Requiring Cryogenic Temperature), Containers (Active Containers, Passive Containers))- Trends, Industry Competition Analysis, Revenue and Forecast To 2030."
According…
More Releases for Viral
Viral Inactivation Market Growing Demand of Kits and Reagents Viral Inactivation …
According to Precision Business Insights (PBI), the latest report, the Viral Inactivation market is expected to be worth USD 2.1 billion in 2022, growing at a 12.8% CAGR from 2022 to 2028. The primary driver of the expansion of the global Viral Inactivation market are speedy growth in pharmaceutical and biotechnology industries and strong inclination of R&D investments in life sciences industry.
View the detailed report description here - https://precisionbusinessinsights.com/market-reports/viral-inactivation-market/
Kits &…
Global Anti-Viral Drug Therapy Market | Global Anti-Viral Drug Therapy Industry …
Anti-viral drug therapy market comprises of sales of branded & generic anti-viral drugs and related services by entities that manufacture the branded or generic anti-viral drugs for treating microbial infections. Anti-viral drug therapy industry includes several establishments that manufacture anti-viral drugs, which are used to treat diseases caused by viral infections. Examples of antiviral drugs include cidofovir, amantadine, zanamivir, idoxuridine, famiciclovir, tenofovir, adefovir and ribavirin.
According to study, “Anti-Viral Drug Therapy…
Viral Antigens Market to Reap Excessive Revenues by 2028(Based on product type, …
Antigens are commonly referred to as substances that are capable of triggering an immune response in a host by activating the lymphocytes or initiating antibody production against infections. Depending upon the fact that they either enter the human body or originate within the body, viral antigens are classified as foreign or self-antigens respectively. Among these, self-antigens comprise mutated or overexpressed proteins. However, foreign antigens are likely to include bacteria, parasites,…
Viral Vector and Plasmid DNA Manufacturing Market - Increase In Awareness Regard …
The Viral Vector and Plasmid DNA Manufacturing Market is expected to reach $ 1,090 million in 2023, growing at an annual average rate of 22.6% from 2017 to 2023. North America was the largest contributor to the viral vector and plasmid DNA markets in 2016. However, the Asia Pacific region is expected to have the highest growth rate during the forecast period.
Gene therapy has included tools such as viral vectors…
Viral Vaccines Market : Challenges and Opportunities Against Viral Diseases
Vaccines are biotic compositions. They are basically formulated from living beings that increase the resistance against a specific illness. Vaccinations that help in avoiding the occurrence of specific diseases are known as prophylactic vaccines and in other instance, the vaccines that are helpful in treating the disease are called therapeutic vaccines. A vaccine is generally produced by using a weakened or murdered type of the sickness causing microorganism. The agent…
Global Viral Molecular Diagnostics Market
The Viral Molecular Diagnostics Market reportprovides in-depth analysis of parent market trends, macro-economic indicators and governing factors along with market attractiveness as per segments. The report also maps the qualitative impact of various market factors on market segments and geographies.
Molecular diagnostics refers to a technique used to detect and identify the presence of genetic material or proteins associated with a specific health condition or disease. Viral molecular diagnostic helps in…