Press release
eClinical Solutions Market Research by Product (CDMS, EDC, CTMS, eCOA, Analytics, RTMS, eTMF, Safety), Delivery Mode, Clinical Trial Phases, End User and Top Competitors Overview
The credible eClinical Solutions Market report comprehensively studies market definition, market segmentation, competitive analysis, and key developments in the market. This report has a lot to offer such as the general market conditions, trends, inclinations, key players, opportunities, geographical analysis, and many other parameters that help to take the business towards growth and success. eClinical Solutions Market document is generated with the comprehension of business goals and needs to bridge the gap by delivering with the most appropriate and suitable solutions; which rises the company’s growth, by subsidizing the risk and improving their performance.Request for FREE Sample Report: To Know the Impact of COVID-19 on this Industry @ https://www.reportsnreports.com/contacts/requestsample.aspx?name=187723
On the basis of clinical trial phases, the global eClinical solutions market is divided into phase I, phase II, phase III, and phase IV. The phase III clinical trials segment accounted for the largest share of the global eClinical solutions market in 2016. In addition, the phase III clinical trials segment is expected to grow at the highest CAGR during the forecast period.
Geographically, North America commanded the largest share of the global eClinical solutions market in 2016, followed by Europe. The large market share of North America is attributed to the significant number of ongoing clinical trials in this region. A number of factors such as the increasing government grants to support clinical trials, continuous product development and launch by eClinical solution vendors, growth in the number of partnerships for new drug development, high prevalence of lifestyle diseases, and an increasing aging population are further stimulating the demand for eClinical solutions in North America. However, strict and lengthy government requirements and high cost of conducting trials are negatively affecting the growth of the market in this region.
Although developed regions such as North America and Europe held large shares in the eClinical solutions market in 2016, it is the Asia-Pacific region that is poised to achieve the highest CAGR in the next five years. Factors such as the increasing outsourcing of clinical trial studies by large pharma and biopharma companies to Asia-Pacific countries, the presence of large patient population, and the low operating cost of conducting clinical trials are propelling the growth of the eClinical solutions market in this region.
The eClinical solutions market is highly competitive, with a large number of global and local players. Oracle Corporation (U.S.) Medidata Solutions, Inc. (U.S.), and PAREXEL International Corporation (U.S.) were the top three players in the eClinical solutions market in 2016. Partnerships, agreements, and collaborations; product launches, enhancements, and deployments; mergers and acquisitions; and geographic expansions are the major strategies adopted by most players to achieve growth in the eClinical solutions market.
Research Coverage:
The report covers software solutions used across all phases in the clinical trial process. It aims at estimating the market size and future growth potential of this market across different segments such as product type, delivery mode, clinical trial phase, end user, and regions. The report also includes an in-depth competitive analysis of the key players with their company profiles, recent developments, and key market strategies.
Reasons to Buy the Report:
This report focuses on various levels of analysis—market share analysis of the top players and company profiles, which discuss basic views on the competitive landscape; emerging and high-growth segments of the eClinical solutions market; and high-growth regions and their respective drivers, restraints, challenges, and opportunities.
The report will enrich both established firms as well as new entrants/smaller firms to gauge the pulse of the market, which, in turn, will help the mariner greater market shares. Companies purchasing the report could use any one of the combination of the strategies mentioned below, namely, market penetration, product development/innovation, market development, market diversification, and competitive assessment to strengthen their market shares.
The report provides insights on the following pointers:
Market Penetration: Comprehensive information on clinical solutions offered by the top 10 players in the market. The report analyzes the eClinical solutions market, by product, delivery mode, clinical trial phase, and end-users across four geographies.
Product Development/Innovation: Detailed insights on current technologies, research and development activities, and product launches in the eClinical solutions market.
Market Development: Comprehensive information of lucrative emerging markets. The report analyzes the markets for various eClinical solutions across four geographies (North America, Europe, Asia-Pacific, and the Rest of the World).
Competitive Assessment: Assessment of market shares, strategies, products, distribution networks, and manufacturing capabilities of the leading players in the eClinical solutions market.
The global eClinical solutions market is estimated to reach 7.61 billion by 2022, at a high CAGR of 12.4% in the forecast period (2017-2022). The growth of the global eClinical solutions market is driven by factors such as a need for improved data standardization and an in creasein R&D expenditure by pharma-biotech companies with significant IT budgets for drug development. In addition, the rising number of clinical trials, especially across emerging countries in the Asia-Pacific region, offers growth opportunities for vendors of eClinical solutions. However, a dearth of skilled research professionals and concerns over patient data privacy are hampering the adoption of these solutions.
Enquire Here for, Report Enquiry, Discount and Customization @ https://www.reportsnreports.com/contacts/inquirybeforebuy.aspx?name=187723
Table of Contents
1 Introduction
1.1 Objectives of the Study
1.2 Market Definition
1.3 Market Scope
1.3.1 Markets Covered
1.3.2 Geographic Scope
1.3.3 Years Considered for the Study
1.4 Currency
1.5 Limitations
1.6 Major Market Stakeholders
2 Research Methodology
2.1 Research Data
2.1.1 Secondary Data
2.1.1.1 Key Data From Secondary Sources
2.1.2 Primary Data
2.1.2.1 Breakdown of Primaries
2.1.2.2 Key Data From Primary Sources
2.1.2.3 Key Industry Insights
2.2 Market Size Estimation
2.2.1.1 Bottom-Up Approach
2.2.1.2 Top-Down Approach
2.3 Market Breakdown and Data Triangulation
2.4 Research Assumptions
3 Executive Summary
4 Premium Insights
4.1 Eclinical Solutions: Market Overview
4.2 Eclinical Solutions Market, By Product, 2015 vs 2015 vs 2022 (USD Million)
4.3 Regional Analysis: Eclinical Solutions Market, By Delivery Mode (2016)
4.4 Eclinical Solutions Market Share, By Clinical Trial Phase (2017 vs 2022)
4.5 Regional Analysis: Eclinical Solutions Market, By End User (2017–2022)
5 Market Overview
5.1 Introduction
5.2 Market Dynamics
5.2.1 Major Market Drivers
5.2.1.1 Increasing Operational Costs and Regulatory Requirements Associated With Clinical Research Studies
5.2.1.2 Growing Adoption of Novel Software Solutions in Clinical Research
5.2.1.3 Rising Government Funding & Grants to Support Clinical Trials
5.2.1.4 Growing Adoption of Eclinical Solutions for Improved Data Standardization
5.2.1.5 Increasing R&D Expenditure By Pharma-Biotech Companies With Augmented It Expenditure Allocations for Drug Development Pipeline
5.2.1.6 Growing Customer Base for Eclinical Solutions
5.2.2 Major Market Restraints
5.2.2.1 High Implementation Costs Associated With Eclinical Solutions
5.2.2.2 Dearth of Skilled Professionals for Operating Eclinical Solutions
5.2.2.3 Limited Awareness Among Researchers Related to Advantages of Eclinical Solutions
5.2.3 Key Growth Opportunities
5.2.3.1 Increasing Clinical Research Activities in Emerging Asian Countries
5.2.3.2 Increased Outsourcing of Clinical Trial Processes By Industrial Researchers to Cros
5.2.3.3 Rising Number of Clinical Trials, Especially Across Emerging Countries
5.2.3.4 Shift From Manual Data Interpretation to Real-Time Data Analysis During Clinical Studies
5.2.4 Major Market Challenges
5.2.4.1 Limited Adoption of Eclinical Solutions in Developing Nations
5.2.4.2 Software Reliability
5.2.4.3 Patient Privacy
6 Global Eclinical Solutions Market, By Product
6.1 Introduction
6.2 Electronic Data Capture and Clinical Data Management Systems
6.3 Clinical Trial Management Systems
6.4 Clinical Analytics Platforms
6.5 Randomization and Trial Supply Management
6.6 Clinical Data Integration Platforms
6.7 Electronic Clinical Outcome Assessment Solutions
6.8 Safety Solutions
6.9 Electronic Trial Master File Systems
6.10 Regulatory Information Management Solutions
6.11 Other Eclinical Solutions
7 Eclinical Solutions Market, By Delivery Mode
7.1 Introduction
7.2 Web-Hosted (On-Demand) Solutions
7.3 Licensed Enterprise (On-Premise) Solutions
7.4 Cloud-Based (SaaS) Solutions
8 Global Eclinical Solutions Market, By Clinical Trial Phase
8.1 Introduction
8.2 Phase I Clinical Trials
8.3 Phase II Clinical Trials
8.4 Phase III Clinical Trials
8.5 Phase IV Clinical Trials
9 Eclinical Solutions Market, By End User
9.1 Introduction
9.2 Pharmaceutical and Biopharmaceutical Companies
9.3 Contract Research Organizations
9.4 Consulting Service Companies
9.5 Medical Device Manufacturers
9.6 Hospitals
9.7 Academic Research Institutes
10 Eclinical Solutions Market, By Region
10.1 Introduction
10.2 North America
10.2.1 U.S.
10.2.2 Canada
10.3 Europe
10.4 Asia-Pacific
10.5 Rest of the World
11 Competitive Landscape
11.1 Overview
11.2 Market Share Analysis
11.3 Competitive Situation and Trends
11.3.1 Agreements, Partnerships, and Collaborations
11.3.2 Product Deployments
11.3.3 Product Launches & Product Enhancements
11.3.4 Mergers & Acquisitions
11.3.5 Expansions
11.3.6 Other Strategies
12 Company Profiles
12.1 Introduction
12.1.1 Geographic Benchmarking
12.2 Oracle Corporation
12.2.1 Business Overview
12.2.2 Products Offered
12.2.3 Recent Developments (2013-2017)
12.2.4 MnM View
12.3 Medidata Solutions, Inc.
12.3.1 Business Overview
12.3.2 Products Offered
12.3.3 Recent Developments (2013-2017)
12.3.4 MnM View
12.4 Parexel International Corporation
12.4.1 Business Overview
12.4.2 Products Offered
12.4.3 Recent Developments (2013-2017)
12.4.4 MnM View
12.5 Bioclinica, Inc. (A Subsidiary of Cinven)
12.5.1 Business Overview
12.5.2 Products Offered
12.5.3 Recent Developments (2013-2017)
12.5.4 MnM View
12.6 Datatrak International, Inc.
12.6.1 Business Overview
12.6.2 Products Offered
12.6.3 Recent Developments (2013-2017)
12.7 CRF Health
12.7.1 Business Overview
12.7.2 Products Offered
12.7.3 Recent Developments (2013-2017)
12.8 ERT Clinical
12.8.1 Business Overview
12.8.2 Products Offered
12.8.3 Recent Developments (2013-2017)
12.9 Merge Healthcare Incorporated
12.9.1 Business Overview
12.9.2 Products Offered
12.9.3 Recent Developments (2013-2017)
12.10 Omnicomm Systems, Inc.
12.10.1 Business Overview
12.10.2 Products Offered
12.10.3 Recent Developments (2013-2017)
12.11 Maxisit Inc.
12.11.1 Business Overview
12.11.2 Products Offered
12.11.3 Recent Developments (2013-2017)
12.12 Bio-Optronics, Inc.
12.12.1 Business Overview
12.12.2 Products Offered
12.12.3 Recent Developments (2013-2017)
12.13 Eclinical Solutions, LLC.
12.13.1 Business Overview
12.13.2 Products Offered
12.13.3 Recent Developments (2013-2017)
13 Appendix
13.1 Discussion Guide*
13.2 Knowledge Store: Marketsandmarkets’ Subscription Portal
13.3 Introducing RT: Real-Time Market Intelligence
13.4 Available Customizations
13.5 Related Reports
Purchase the Report Directly @ https://www.reportsnreports.com/purchase.aspx?name=187723
Contact Us:
Corporate Headquarters
Tower B5, office 101,
Magarpatta SEZ,
Hadapsar, Pune-411013, India
+ 1 888 391 5441
sales@reportsandreports.com
About Us:
ReportsnReports.com is your single source for all market research needs. Our database includes 500,000+ market research reports from over 95 leading global publishers & in-depth market research studies of over 5000 micro markets. With comprehensive information about the publishers and the industries for which they publish market research reports, we help you in your purchase decision by mapping your information needs with our huge collection of reports.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release eClinical Solutions Market Research by Product (CDMS, EDC, CTMS, eCOA, Analytics, RTMS, eTMF, Safety), Delivery Mode, Clinical Trial Phases, End User and Top Competitors Overview here
News-ID: 2318409 • Views: …
More Releases from ReportsnReports
DeviceCon Series 2024 - UK Edition | MarketsandMarkets
Future Forward: Redefining Healthcare with Cutting-Edge Devices
Welcome to DeviceCon Series 2024 - Where Innovation Meets Impact!
Join us on March 21-22 at Millennium Gloucester Hotel, 4-18 Harrington Gardens, London SW7 4LH for a groundbreaking convergence of knowledge, ideas, and technology. MarketsandMarkets proudly presents the DeviceCon Series, an extraordinary blend of four conferences that promise to redefine the landscape of innovation in medical and diagnostic devices.
Register Now @ https://events.marketsandmarkets.com/devicecon-series-uk-edition-2024/register
MarketsandMarkets presents…

5th Annual MarketsandMarkets Infectious Disease and Molecular Diagnostics Confer …
London, March 7, 2024 - MarketsandMarkets is thrilled to announce the eagerly awaited 5th Annual Infectious Disease and Molecular Diagnostics Conference, scheduled to take place on March 21st - 22nd, 2024, at the prestigious Millennium Gloucester Hotel, located at 4-18 Harrington Gardens, London SW7 4LH.
This conference promises to be a groundbreaking event, showcasing the latest trends and insights in diagnosis, as well as unveiling cutting-edge technologies that are revolutionizing the…

Infection Control, Sterilization & Decontamination Conference |21st - 22nd March …
MarketsandMarkets is pleased to announce its 8th Annual Infection Control, Sterilisation, and Decontamination in Healthcare Conference, which will take place March 21-22, 2024, in London, UK. With the increased risk of infection due to improper sterilisation and decontamination practices, the safety of patients and healthcare workers is of paramount importance nowadays.
Enquire Now @ https://events.marketsandmarkets.com/infection-control-sterilization-and-decontamination-conference/
This conference aims to bring together all the stakeholders to discuss the obstacles in achieving…
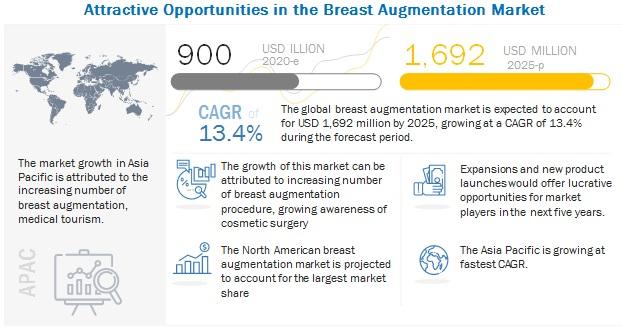
Breast Augmentation Market Key Players, Demands, Cost, Size, Procedure, Shape, S …
The global Breast Augmentation Market in terms of revenue was estimated to be worth $900 million in 2020 and is poised to reach $1,692 million by 2025, growing at a CAGR of 13.4% from 2020 to 2025. The new research study consists of an industry trend analysis of the market. The new research study consists of industry trends, pricing analysis, patent analysis, conference and webinar materials, key stakeholders, and buying…
More Releases for Clinical
Clinical Laboratory Market in Indonesia, Clinical Laboratory Industry in Indones …
"Increase in healthcare expenditure from the Indonesian government has driven the growth of clinical laboratory market in Indonesia."
Increase in Healthcare Awareness: Largely driven by increase in healthcare spending by aging population (~$ 260 per person by 2050), rising income levels, rising awareness for preventive testing, advanced healthcare diagnostic tests offerings, and central government's healthcare measures.
Developments in Testing and Preference for Evidence based testing: There is also a rising number…
Clinical solutions
Are you spending more time with yellow files than with patients? Healthbridge can change that with our intuitive and easy to use clinical platform that is designed specifically for the medical practitioner at the practice.
smaller2
Easily access patient
information
Cloud-based technology enables you to store rich clinical information that can be easily accessed as and when you need it.
Medical billing software innovation
Become a paperless
practice
Create scripts, sick notes, and clinical notes electronically. Plus, have…
Clinical Research and Clinical Trials Summit
Clinical Research 2019 has been designed in an interdisciplinary manner with a multitude of tracks to choose from every segment and provides you with a unique opportunity to meet up with peers from both industry and academia and establish a scientific network between them. We cordially invite all concerned people to come join us at our event and make it successful by your participation.
This is the premier interdisciplinary forum for…
Paediatric Clinical Trial Conference - When designing a Paediatric clinical tria …
Press Release – 12.02.2018
When designing a Paediatric clinical trial, a paediatric investigation plan (PIP) is a development plan aimed at ensuring that the necessary data are obtained through studies in children, to support the authorisation of a medicine for children. All applications for marketing authorisation for new medicines have to include the results of studies as described in an agreed PIP, unless the medicine is exempt because of a deferral…
Clinical Communication
According to a recent market report published by Persistence Market Research titled “Clinical Communication and Collaboration Market: Global Industry Analysis (2012–2016) and Forecast (2017–2025),” revenue from the global clinical communication and collaboration market was US$ 138.5 Mn in 2012 and US$ 214.8 Mn in 2016, representing a CAGR of 11.6% from 2012 to 2016. This revenue growth is attributed to addition of new features in clinical communication and collaboration solutions.…
E-Clinical Trial Solutions Market To Accelerating Clinical Development Technolog …
The study of the "Global e-Clinical Trial Solutions Market" provides the market size information and market trends along with the factors and parameters impacting it in both short and long term. The study ensures a 360° view, bringing out the complete key insights of the industry.
The Global e-Clinical Trial Solutions Market Research Report Forecast 2017-2021 is a valuable source of insightful data for business strategists. It provides the e-Clinical…