Press release
Siora Surgicals Proudly Announces ISO 13485:2016 Certification
A step towards producing high-quality orthopedic implants and building trustworthy relationships.Getting the ISO 13485:2016 certification is an important landmark for Siora Surgicals. It shows our commitment to provide international standard quality orthopedic implants that meet customer expectations. We will continue to develop components that are of great quality and conform to the highest standards. ISO 13485:2016 certification completely justifies our last statement and we are proud to get this.
ISO 13485 certification ensures that the quality management system of the Siora Surgicals is effective enough to produce world-class products with its consistent research & development, design & development, production, and sale of medical devices that are safe to use. This certification also builds trust about the consistency and precision in the design & quality of products manufactured.
Siora Surgicals Pvt. Ltd. has always urged for quality rather than quantity, and that is what has helped the company grow and mark its reputable position in the national as well as international market. Our true quality products are praised in the market and are used by top orthopedic surgeons across the globe. Achieving this ISO certification is a great accomplishment by our team and an excellent example of Siora’s commitment to delivering the highest levels of quality and regulatory compliance to our clients. Siora has been a consistent performer in terms of its quality and commitment to customer satisfaction. We believe in building trust with the clients and ensure them that they are using the safest products in the market. The company manufactures a wide range of orthopedic devices including Hand Fracture Plate (https://www.siiora.com/locking-hand-system/) ,Locking Compression Plate, and Interlocking Intramedullary Nail.
Certain benefits of implementing ISO 13485:2016 include:
Leading and leaving with the international standards in the global medical device community. An ISO-certified company is well versed with the system, culture, and ethics of another ISO-certified company, and hence both can work hand in hand.
In the medical device industry, ISO 13485 is known to be the gold standard for quality. Certification to this standard confirms that the company is serious about the quality of products it delivers and to ensure that the company has a well-established system in place.
The ISO 13485 standard has a set of quality management principles and that ensures customer satisfaction.
Gives a chance to work with top players in the market as they only want to work with ISO-certified companies.
Shows consistent commitment to the quality, safety, efficacy, performance while production, and delivery of high-quality products.
ISO 13485 shows that Siora complies with medical device regulations and legal requirements, eliminating uncertainty for all stakeholders.
ISO 13485:2016 documentation provides the latest & relevant information to all the team members of the development team while reducing the time & cost associated with the product development.
Boosts company knowledge while helping them to easily identify the problems and improve the development process.
Provides more opportunities to get a global market presence and company growth.
Besides all this, Siora Surgicals Pvt. Ltd. has also received ISO 9001:2015 and this ensures that the quality management system of the company is established according to all stated guidelines. This certification helps companies focus on the key areas and assist in improving the efficiency of the whole system. Being the most recognized Quality Management System (QMS) standard of the world, ISO 9001 is based on 7 quality management principles:
Customer Focus
Leadership
Engagement of people
Process approach
Improvement
Evidence-based decision making
Relationship management
This is a flexible standard that helps organizations define their objectives and streamline the process to achieve them with maximum output. Siora Surgicals Pvt. Ltd. is a reliable Orthopedic Implants exporter in Romania (https://www.siiora.com/romania/ )and ISO certifications received by the company completely justifies that.
Siora Surgicals Private Limited
WZ- 1, 2nd Floor, Phool Bagh, Ram Pura, New Delhi, 110035 INDIA
+91 9810021264
Siora Surgicals Pvt. Ltd. is one of the Leading orthopedic implants and instruments manufacturing companies in India. Siora Surgicals Pvt. Ltd. manufactures and export orthopedic implants and instruments for over three decades. We have a comprehensive portfolio of trauma products numbering more than two thousand. Our Corporate Office is in Delhi and the manufacturing unit is in Sonipat Rai Industries.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Siora Surgicals Proudly Announces ISO 13485:2016 Certification here
News-ID: 2268616 • Views: …
More Releases from Siora Surgicals Pvt Ltd
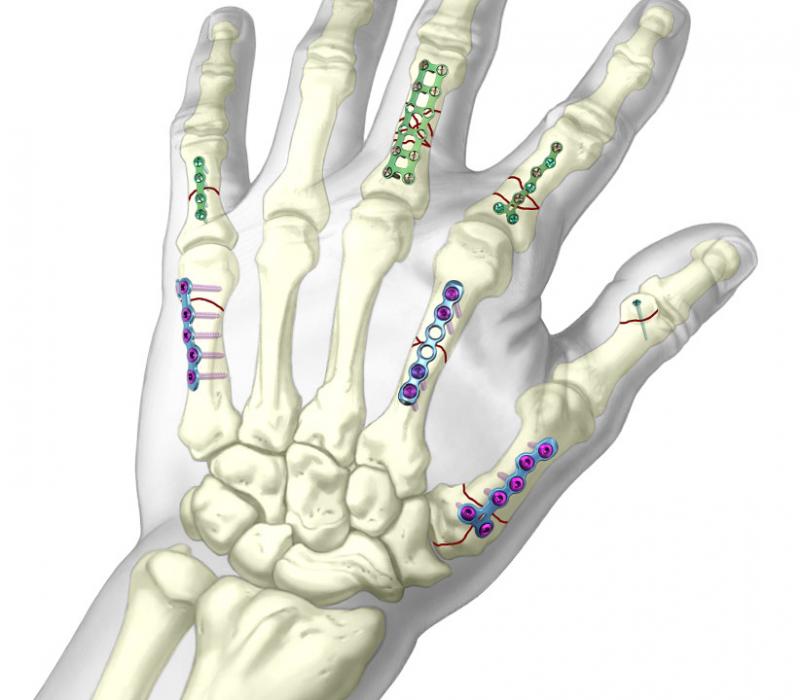
Siora Surgicals Introduces Wide Range of Microlock Locking Hand Plate
Microlock Locking hand System is designed for the small bone of the hand fractures.
Locking hand plates are fracture fixation devices with threaded screw holes. The system consists of 40 different plates, available in stainless steel and titanium. Locking hand plates (https://www.siiora.com/locking-hand-system/) are used for the different types of fractures like Stable fracture, Unstable fracture, Comminuted fracture, Open or compound fracture.
The bones of the hand are the supporting structure of the…
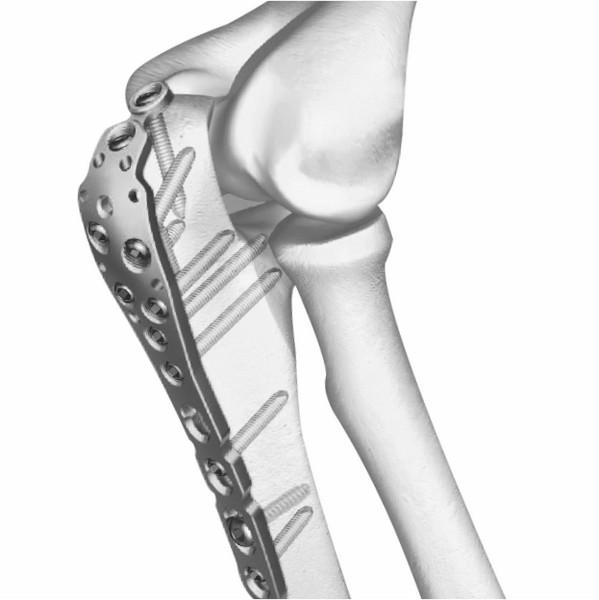
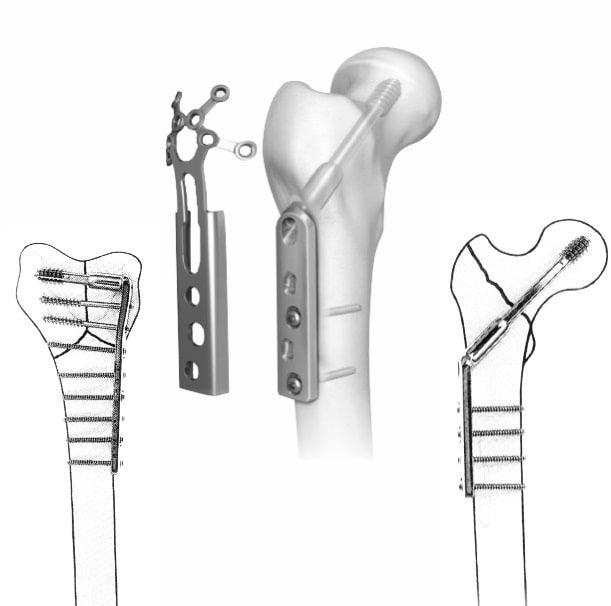
Siora Surgicals Introduces ISO Certified Locking Plates
“Siora Surgicals launches various ranges of locking plates for small and large bone fixation.”
Siora Surgicals Pvt. Ltd., one of the oldest orthopedic implants company, has introduced its new line of locking plate (https://www.siiora.com/locking-plates/) , distal radius plate, and locking hand plates. Like all other implant products in the Siora catalog, the fragments of these locking implants are individually tested for durability and stability. This addition to the company’s existing…
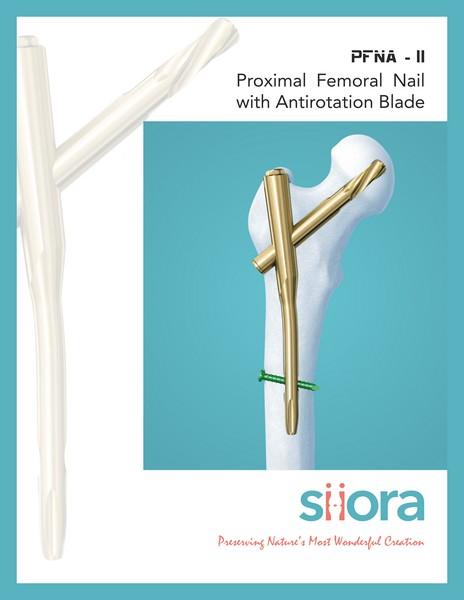
Siora Surgicals Introduces Wide Range of Intramedullary Interlocking Nail
"An intramedullary Nails (IM Nail), also known as intramedullary rod or inter-locking nail, is a metal rod pushed into the medullary cavity of a bone."
Interlocking Nail (https://www.siiora.com/interlocking-nails/) has been used for the treatment of long bone fractures of the body. IM nails are used for the different types of fractures like Fibula Fracture, Tibia Fracture, and femur fracture, etc.
Fibula Fracture: A fibula fracture can happen when there is an injury…

Meet Siora Surgicals at IOACON 2019
Siora Surgicals is well-positioned to become a leader in the rapidly evolving medical implant manufacturing companies in India. We are committed and dedicated to our continuous research and development process, to build and maintain a robust product system. In the Indian orthopedic association conference (IOACON 2019) Siora Surgical Pvt Ltd, One of the leading orthopedic implants and instrument manufacturers participating with the aim to give people a more active, productive…
More Releases for ISO
Layer3 achieves ISO 27001 and ISO 27017 Certifications
Layer3, an industry leader in cloud, scalable and secure networks, has achieved the much-in-demand ISO/IEC 27001:2013 and ISO/IEC 27017:2015 certifications.
ISO/IEC 27001:2013 is the most widely used information security standard, prepared and published by the International Organization for Standardization (ISO), the world’s largest developer of voluntary international standards. It is a globally recognized standard mandating numerous controls for the establishment, maintenance, and certification of an information security management system (ISMS). The…
ISO Certification
CRMIT Solutions has announced that it has been awarded the ISO/IEC 27001:2013 certification, which will see further support of the company's digital transformation solutions, products and services business.
Bangalore, KA (May 21, 2020)- CRMIT Solutions, a pioneer in digital transformation and Customer360 solutions, have been re-confirmed the ISO/IEC 27001:2013 certification after the company successfully developed and implemented an integrated strategy for information security management to protect information assets, such as customer,…
ISO Certification Market Report 2018: Segmentation by Type (ISO 9001:2015, ISO 2 …
Global ISO Certification market research report provides company profile for The British Standards Institution, CERTIFICATION EUROPE, NQA, Lakshy Management Consultant, URS Holdings, Bureau Veritas, DNV GL AS (International Standards Certifications Global FZ), SGS, Lloyd's Register Group Services, Intertek and Others.
This market study includes data about consumer perspective, comprehensive analysis, statistics, market share, company performances (Stocks), historical analysis 2012 to 2017, market forecast 2018 to 2025 in terms of volume,…
ISO 27001/ISO 27002 Consultancy,ISO 27000,ISO 27000 Consultancy,Information Secu …
Coralesecure is a Information Security Management System (ISMS) – ISO 27001 Compliance. ISO 27000 Consultancy deals with maintaining the integrity and availability of organizational information and knowledge. Information Security Management System provides the experts on business management, and information security support and properly engages in executive communications Data loss, whether through cyber attacks or other forms of malicious intent can quickly bring an organization to its knees? The protection of…
ISO 27001 India, 27001 training India ISO
Coral eSecure is information Risk Management advisory with specific focus on Information Security (ISO 27001, ISO 17799, PCI, COBIT, HIPAA, GLBA, and DPA), Business Continuity (BS25999) and ITIL/ IT Service Management (ISO 20000). Coral is the FIRST Indian consulting organization which provides an Integrated Management System Consulting, Consisting of ISO 27001, ISO 20000 and BS25999 – ALL THREE IN ONE! Coral provides Consulting, Assurance audit and Training to address these…
ISO 27001 india, 27001 training india ISO, 17799 training india ISO, Compliance …
Our foundation of service delivery is based on sound research - thereby customers realizing definitive delight and Assurance resilience in their management system. We provide Consulting Services for ISO 27001 india, 27001 training india ISO,17799 training India ISO Security, BCM/BS25999, SAS 70, COBIT Implementation, Annual Compliance Check, Best Practices Implementation, & Quick Gap Analysis. It is a set of ‘best practices’ controls - management and systems - that enables…