Press release
CTLA-4 Inhibitors Competitive Landscape and Clinical Trials Assessment 2024: FDA Approvals, Therapies and Key Companies involved by DelveInsight | Henlix Biotech, Harbour BioMed, AstraZeneca, Innovent
(Las Vegas, Nevada, United States) As per DelveInsight's assessment, globally, CTLA-4 Inhibitors Competitive Landscape constitutes 40+ key companies continuously working towards developing 50+ CTLA-4 inhibitors treatment therapies, analysis of Clinical Trials, Therapies, Mechanism of Action, Route of Administration, and Developments analyzes DelveInsight.The CTLA-4 Inhibitors Competitive Landscape report embraces in-depth commercial and clinical assessment of the pipeline products from the pre-clinical developmental phase to the marketed phase. The report also covers a detailed description of the drug, including the mechanism of action of the drug, clinical studies, NDA approvals (if any), and product development activities comprising the technology, collaborations, mergers acquisition, funding, designations, and other product-related details.
"CTLA-4 Inhibitors Competitive Landscape Insight, 2023 [https://www.delveinsight.com/sample-request/ctl-4-inhibitors-competitive-landscape?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=gpr]" report by DelveInsight outlines comprehensive insights into the present clinical development scenario and growth prospects across the CTLA-4 inhibitors Market.
Some of the key takeaways from the CTLA-4 Inhibitors Competitive Landscape Report:
*
Companies across the globe are diligently working toward developing novel CTLA-4 inhibitors treatment therapies with a considerable amount of success over the years.
*
CTLA-4 inhibitors companies working in the treatment market are Molecular Templates, Shanghai Henlius Biotech, Harbour BioMed, Alpine Immune Sciences, Agenus, Xencor, Innovent Biologics, Alphamab Oncology, Bristol-Myers Squibb, Akeso Biopharma, and others, are developing therapies for the CTLA-4 inhibitors treatment
*
Emerging CTLA-4 inhibitors therapies in the different phases of clinical trials are- MT-8421, HLX-13, HBM4003, ALPN-202, Botensilimab, Vudalimab, IBI-310, KN046, Ipilimumab, Cadonilimab, and others are expected to have a significant impact on the CTLA-4 inhibitors market in the coming years.
*
In March 2023, Molecular Templates, Inc. obtained approval from the US Food and Drug Administration (FDA) after evaluating its Investigational New Drug Application (IND) to advance clinical trials for its innovative MT-8421 ETB program, which targets CTLA-4 in patients with relapsed/refractory solid tumors previously treated with checkpoint inhibitors. Preclinical findings from MT-8421 revealed that treatment depleted immune suppressive Tregs in the TME but not in the periphery, as observed in a transgenic mouse model expressing human CTLA-4 and harboring syngeneic subcutaneous tumors.
*
In March 2023, Agenus initiated a randomized, open-label, Phase II Trial of Nab-paclitaxel +Gemcitabine with or without Botensilimab (AGEN1181) in patients with metastatic pancreatic cancer who have progressedon prior 5FU + Leucovorin + Irinotecan + Oxaliplatin (FOLFIRINOX). This will be a prospective, multicenter, clinical trial ofbotensilimab in combination with nab-paclitaxel + gemcitabine or nab-paclitaxel + gemcitabine alone.
*
In February 2023, Akeso Biopharma Co., Ltd., in partnership with Chia Tai Tianqing Pharmaceutical Group Co., Ltd., has launched a clinical trial for Cadonilimab Plus Anlotinib in patients with recurrent, metastatic, or persistent cervical cancer. This trial aims to evaluate the efficacy of a novel treatment regimen comprising cadonilimab and anlotinib. Currently, the trial is actively enrolling participants and is anticipated to conclude by October 2024, with an expected enrollment of 48 participants.
*
In October 2022, The FDA greenlit tremelimumab (Imjudo), a CTLA-4 immune checkpoint inhibitor, for use in combination with durvalumab (Imfinzi) as a first-line immunotherapy for unresectable hepatocellular cancer (HCC). This new indication involves administering a single 300 mg priming dose of tremelimumab alongside durvalumab at a dose of 1,500 mg every 4 weeks, known as the STRIDE regimen (single tremelimumab, regular interval durvalumab).
*
In August 2022, Biocytogen Pharmaceuticals and TRACON Pharmaceuticals, Inc. revealed that the FDA granted approval for the Investigational New Drug (IND) application, allowing the commencement of a Phase I/II clinical trial for YH001 combined with envafolimab and doxorubicin. This study aims to evaluate the treatment's efficacy in sarcoma patients, including those who have not undergone prior therapy.
CTLA-4 inhibitors Overview
CTLA-4 inhibitors, or cytotoxic T-lymphocyte-associated protein 4 inhibitors, are a class of immunotherapy drugs used in cancer treatment. CTLA-4 is a protein receptor found on the surface of T cells, a type of immune cell. It regulates the activity of T cells and helps prevent them from attacking healthy cells in the body. However, cancer cells can exploit this mechanism to evade the immune system.
Get a Free Sample PDF Report to know more about CTLA-4 Inhibitors Competitive Landscape Therapeutic Assessment-
https://www.delveinsight.com/report-store/ctl-4-inhibitors-competitive-landscape [https://www.delveinsight.com/report-store/ctl-4-inhibitors-competitive-landscape?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=gpr]
Emerging CTLA-4 inhibitors Drugs Under Different Phases of Clinical Development Include:
*
MT-8421: Molecular Templates
*
HLX-13: Shanghai Henlius Biotech
*
HBM4003: Harbour BioMed
*
ALPN-202: Alpine Immune Sciences
*
Botensilimab: Agenus
*
Vudalimab: Xencor
*
IBI-310: Innovent Biologics
*
KN046: Alphamab Oncology
*
Ipilimumab: Bristol-Myers Squibb
*
Cadonilimab: Akeso Biopharma
CTLA-4 inhibitors Route of Administration
CTLA-4 Inhibitors Competitive Landscape report provides the therapeutic assessment of the pipeline drugs by the Route of Administration. Products have been categorized under various ROAs, such as
*
Oral
*
Parenteral
*
Intravenous
*
Subcutaneous
*
Topical
CTLA-4 inhibitors Molecule Type
CTLA-4 inhibitors Products have been categorized under various Molecule types, such as
*
Monoclonal Antibody
*
Peptides
*
Polymer
*
Small molecule
*
Gene therapy
CTLA-4 Inhibitors Competitive Landscape Therapeutics Assessment
*
CTLA-4 inhibitors Assessment by Product Type
*
CTLA-4 inhibitors By Stage and Product Type
*
CTLA-4 inhibitors Assessment by Route of Administration
*
CTLA-4 inhibitors By Stage and Route of Administration
*
CTLA-4 inhibitors Assessment by Molecule Type
*
CTLA-4 inhibitors by Stage and Molecule Type
DelveInsight's CTLA-4 inhibitors Report covers around 50+ products under different phases of clinical development like
*
Late-stage products (Phase III)
*
Mid-stage products (Phase II)
*
Early-stage product (Phase I)
*
Pre-clinical and Discovery stage candidates
*
Discontinued & Inactive candidates
*
Route of Administration
Further CTLA-4 inhibitors product details are provided in the report. Download the CTLA-4 Inhibitors Competitive Landscape report to learn more about the emerging CTLA-4 inhibitors therapies [https://www.delveinsight.com/sample-request/ctl-4-inhibitors-competitive-landscape?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=gpr]
Some of the key companies in the CTLA-4 inhibitors Therapeutics Market include:
Key companies developing therapies for CTLA-4 inhibitors are - Innovent Biologics, Alphamab Oncology, Agenus, Harbour BioMed, AstraZeneca, Alpine Immune Sciences, Henlix Biotech, DotBio, TrueBinding, Ocean Biomedical, Xencor, Molecular Templates, Akeso, Inc., TRACON Pharmaceuticals, Inc., Biocytogen Pharmaceuticals, and others.
CTLA-4 Inhibitors Competitive Landscape Analysis:
The CTLA-4 Inhibitors Competitive Landscape report provides insights into
*
The report provides detailed insights about companies that are developing therapies for the treatment of CTLA-4 inhibitors with aggregate therapies developed by each company for the same.
*
It accesses the Different therapeutic candidates segmented into early-stage, mid-stage, and late-stage of development for CTLA-4 inhibitors Treatment.
*
CTLA-4 inhibitors key companies are involved in targeted therapeutics development with respective active and inactive (dormant or discontinued) projects.
*
CTLA-4 inhibitors Drugs under development based on the stage of development, route of administration, target receptor, monotherapy or combination therapy, a different mechanism of action, and molecular type.
*
Detailed analysis of collaborations (company-company collaborations and company-academia collaborations), licensing agreement and financing details for future advancement of the CTLA-4 inhibitors market.
The report is built using data and information traced from the researcher's proprietary databases, company/university websites, clinical trial registries, conferences, SEC filings, investor presentations, and featured press releases from company/university websites and industry-specific third-party sources, etc.
Download Sample PDF Report to know more about CTLA-4 inhibitors drugs and therapies [https://www.delveinsight.com/sample-request/ctl-4-inhibitors-competitive-landscape?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=gpr]
CTLA-4 Inhibitors Competitive Landscape Market Drivers
*
Rise in prevalence of Cancer, increased focus on the applications ofImmunotherapy using CTLA-4 inhibitors for oncology settings are some of the important factors that are fueling the CTLA-4 inhibitors Market.
CTLA-4 Inhibitors Competitive Landscape Market Barriers
*
However, side-effects associated with the checkpoint inhibitors immunotherapy, high cost associated with the Treatment and other factors are creating obstacles in the CTLA-4 inhibitors Market growth.
Scope of CTLA-4 Inhibitors Competitive Landscape Drug Insight
*
Coverage: Global
*
Key CTLA-4 inhibitors Companies: Molecular Templates, Shanghai Henlius Biotech, Harbour BioMed, Alpine Immune Sciences, Agenus, Xencor, Innovent Biologics, Alphamab Oncology, Bristol-Myers Squibb, Akeso Biopharma, and others
*
Key CTLA-4 inhibitors Therapies: MT-8421, HLX-13, HBM4003, ALPN-202, Botensilimab, Vudalimab, IBI-310, KN046, Ipilimumab, Cadonilimab, and others
*
CTLA-4 inhibitors Therapeutic Assessment: CTLA-4 inhibitors current marketed and CTLA-4 inhibitors emerging therapies
*
CTLA-4 inhibitors Market Dynamics: CTLA-4 inhibitors market drivers and CTLA-4 inhibitors market barriers
Request for Sample PDF Report for CTLA-4 Inhibitors Competitive Landscape Assessment and clinical trials [https://www.delveinsight.com/sample-request/ctl-4-inhibitors-competitive-landscape?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=gpr]
Table of Contents
1. CTLA-4 inhibitors Report Introduction
2. CTLA-4 inhibitors Executive Summary
3. CTLA-4 inhibitors Overview
4. CTLA-4 inhibitors- Analytical Perspective In-depth Commercial Assessment
5. CTLA-4 Inhibitors Competitive Landscape Therapeutics
6. CTLA-4 inhibitors Late Stage Products (Phase II/III)
7. CTLA-4 inhibitors Mid Stage Products (Phase II)
8. CTLA-4 inhibitors Early Stage Products (Phase I)
9. CTLA-4 inhibitors Preclinical Stage Products
10. CTLA-4 inhibitors Therapeutics Assessment
11. CTLA-4 inhibitors Inactive Products
12. Company-University Collaborations (Licensing/Partnering) Analysis
13. CTLA-4 inhibitors Key Companies
14. CTLA-4 inhibitors Key Products
15. CTLA-4 inhibitors Unmet Needs
16 . CTLA-4 inhibitors Market Drivers and Barriers
17. CTLA-4 inhibitors Future Perspectives and Conclusion
18. CTLA-4 inhibitors Analyst Views
19. Appendix
20. About DelveInsight
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences. It supports Pharma companies by providing comprehensive end-to-end solutions to improve their performance. It also offers Healthcare Consulting Services, which benefits in market analysis to accelerate business growth and overcome challenges with a practical approach.
Media Contact
Company Name: DelveInsight Business Research LLP
Contact Person: Gaurav Bora
Email:Send Email [https://www.abnewswire.com/email_contact_us.php?pr=ctla4-inhibitors-competitive-landscape-and-clinical-trials-assessment-2024-fda-approvals-therapies-and-key-companies-involved-by-delveinsight-henlix-biotech-harbour-biomed-astrazeneca-innovent]
Phone: +14699457679
Address:304 S. Jones Blvd #2432
City: Las Vegas
State: NV
Country: United States
Website: https://www.delveinsight.com/
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release CTLA-4 Inhibitors Competitive Landscape and Clinical Trials Assessment 2024: FDA Approvals, Therapies and Key Companies involved by DelveInsight | Henlix Biotech, Harbour BioMed, AstraZeneca, Innovent here
News-ID: 3492850 • Views: …
More Releases from ABNewswire
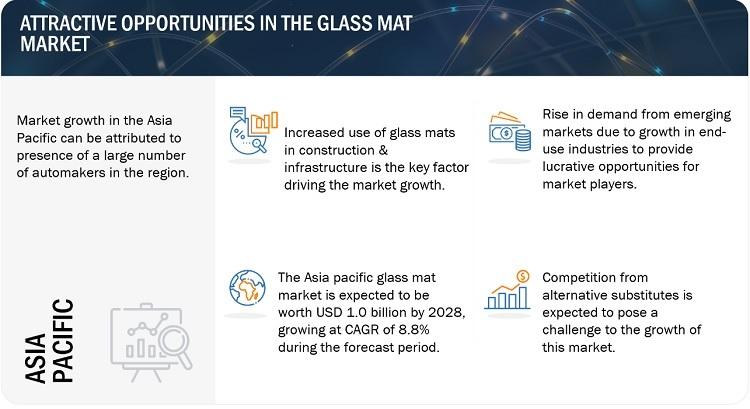
Glass Mat MarketSize, Growth, Opportunities, Top Manufacturers, Share, Trends, S …
Glass Mat Market by Mat Type (Chopped Strand Mat, Continous Filament Mat), Binder Type (Emulsion, Powder), Manufacturing Process (Dry-Laid, Wet-Laid), End-Use Industry, Region (North America, APAC, Europe, Latin America, MEA) - Global Forecast to 2028
The glass mat market [https://www.marketsandmarkets.com/Market-Reports/glass-mat-market-32320559.html] size is estimated to be USD 1.3 billion in 2022 and is projected to grow at a CAGR of 8.6% between 2023 to 2028. The use of glass mat in the…

Chazin & Company Announces Three Grants to Arizona Nonprofits as Part of its Ina …
Mission driven financial solutions experts shows solidarity with Arizona community organizations.
Chazin & Company [https://www.chazinandcompany.com/], a leading provider of outsourced accounting and finance services for nonprofit organizations nationwide, today announced it has awarded three $5,000 unrestricted grants to Arizona nonprofits as part of its first-ever Roadshow event.
The May 20 Chazin Roadshow in Arizona is the first event in a series that Chazin & Company plans to hold around the country. Organizers…

YADAH Leads the Vegan Beauty Revolution with High-Quality, Eco-Friendly Products
Image: https://www.abnewswire.com/uploads/a226cf602a97874c8bfe654d3527841e.png
28th May, 2024 - Amidst the growing popularity of vegan cosmetics in the beauty market, YADAH (YADAH, CEO Kang Eun-mi) is at the forefront of the vegan beauty trend by developing products with high-quality ingredients that are not harmful to the skin.
Since its establishment in 2011, YADAH has consistently avoided the use of animal-derived or tested ingredients, harmful substances, even before "vegan beauty" became a trend. The company has…

Global Aircraft Electric Motors Market Set to Reach New Heights by 2027
The Global Aircraft Electric Motors Market Size is projected to grow from USD 8.2 billion in 2022 to USD 12.9 billion by 2027, at a CAGR of 9.4% from 2022 to 2027.
The global Aircraft Electric Motors [https://www.marketsandmarkets.com/Market-Reports/aircraft-electric-motors-market-3248447.html] Market is poised for significant growth, driven by advancements in electric propulsion technology and increasing demand for more efficient and environmentally friendly aircraft. According to a comprehensive report by MarketsandMarkets, titled "The Global…
More Releases for Inhibitors
Immune Checkpoint Inhibitors Market: Current Evidence And Future Perspectives Of …
Stratagem Market Insights delivers key insights for the Immune Checkpoint Inhibitors market in its published report, which include global industry analysis, Size, Growth, Opportunities, Emerging Trends, Challenges, and Geographic Regions over the forecast period (2021-2028). In terms of revenue, the global Immune Checkpoint Inhibitors market is projected to grow at a CAGR of XX% during the forecast period, owing to several factors about which SMI offers detailed insights and forecasts…
Vesicular Monoamine Transporter-2 Inhibitors (VMAT2 Inhibitors) Market to Regist …
Vesicular monoamine transporter 2 (VMAT2) is a membrane-embedded protein that is responsible for uptake of monoamines neurotransmitters such as dopamine, serotonin, norepinephrine, and histamines into the synaptic vesicles in monoaminergic neurons. Vesicular monoamine transporter proteins 2 are expressed in the central nervous system (CNS) and plays the crucial role in the signaling process between the monoamine neurons. VMAT2 is the only transporter that carries the cytoplasmic dopamine into the vesicles.…
Crude Oil Flow Improvers Market by Type (Paraffin Inhibitors, Asphaltene Inhibit …
Global Crude Oil Flow Improvers Market was valued at $1,282 million in 2016, and is anticipated to reach $1,920 million, growing at a CAGR of 5.7% from 2017 to 2023. Crude oil is one of the most actively traded commodities with a stable growth rate globally. It is extracted from remote locations and needs to be transported through pipelines. Transportation of crude oil via pipeline is relatively difficult, owing to…
Immune Check Point Inhibitors Market Immune Check Point Inhibitors Clinical Pipe …
For Report Sample Contact: neeraj@kuickresearch.com or +91-11-47067990
Report Table of Contents
Immune Checkpoint Inhibitors- Introduction to Next Generation Cancer Immunotherapy
1.1 Overview
1.2 History- From Tragedy to Breakthrough
Major Immune Checkpoints Proteins
2.1 Role of Cytotoxic T lymphocyte Antigen-4 (CTLA-4) in Cancer Development
2.2 Role of Programed Cell Death Protein 1 (PD1) in Cancer Development
Mechanism of Action of Immune Checkpoint Inhibitors
3.1 Cytotoxic T lymphocyte Antigen-4…
Global Immune Check Point Inhibitors Market & Immune Check Point Inhibitors Clin …
Immunotherapy represents a paradigm shift in oncology therapy along the multiple fronts. Although, targeted therapy upgrades the underlying signaling defect which results in oncogenesis within the tumor. The immune checkpoint blockade is fundamentally a therapy which directed at the patient's native immune system to tilt the immune homeostasis away from self-tolerance towards cytotoxicity, with the goal of inducing antitumor immunity.
The immune checkpoint inhibitors such as ipilimumab, nivolumab and pembrolizumab…
Tumor Necrosis Factor Inhibitors Market & TNF Inhibitors Clinical Pipeline Outlo …
The search for the effective therapeutic approaches in the modulation of the TNF has been focus of the research efforts. Approximately 1 Million people in the worldwide either undergoing the treatment or have been treated with the TNF inhibitors which are available in the pharmaceutical market which surrounding the indications that include the rheumatoid arthritis, psoriatic arthritis, psoriasis and inflammatory bowel diseases.
The neutralization of the TNF signaling can be achieved…
