Press release
Benign Prostatic Hyperplasia Pipeline Drugs Analysis Report, 2024 Updates: FDA Approvals, Clinical Trials, Therapies, MOA, ROA by DelveInsight | Boehringer Ingelheim, Merck & Co. Inc., Allergan PLC
(Las Vegas, Nevada, United States) As per DelveInsight's assessment, globally, Benign Prostatic Hyperplasia pipeline constitutes 7+ key companies continuously working towards developing 8+ Benign Prostatic Hyperplasia treatment therapies, analysis of Clinical Trials, Therapies, Mechanism of Action, Route of Administration, and Developments analyzes DelveInsight.The Benign Prostatic Hyperplasia Pipeline report embraces in-depth commercial and clinical assessment of the pipeline products from the pre-clinical developmental phase to the marketed phase. The report also covers a detailed description of the drug, including the mechanism of action of the drug, clinical studies, NDA approvals (if any), and product development activities comprising the technology, collaborations, mergers acquisition, funding, designations, and other product-related details.
"Benign Prostatic Hyperplasia Pipeline Insight, 2024 [https://www.delveinsight.com/sample-request/benign-prostatic-hyperplasia-bph-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=gpr]" report by DelveInsight outlines comprehensive insights into the present clinical development scenario and growth prospects across the Benign Prostatic Hyperplasia Market.
Some of the key takeaways from the Benign Prostatic Hyperplasia Pipeline Report:
*
Companies across the globe are diligently working toward developing novel Benign Prostatic Hyperplasia treatment therapies with a considerable amount of success over the years.
*
Benign Prostatic Hyperplasia companies working in the treatment market are AiViva BioPharma, Chong Kun Dang, EMS, Urotronic Inc., Resurge Therapeutics Inc, Antev, Nymox Pharmaceutical, GlaxoSmithKline, Dongkook Pharmaceutical, RECORDATI GROUP, Astellas Pharma Inc, Eli Lilly and Company, Sophiris Bio Corp, Bayer, Sophiris Bio Corp, Warner Chilcott, Nymox Corporation, QLT Inc., NeoTract, Inc., Xintian Pharmaceutical, and others, are developing therapies for the Benign Prostatic Hyperplasia treatment
*
Emerging Benign Prostatic Hyperplasia therapies in the different phases of clinical trials are- AIV007, CKD-846, DTT106, Paclitaxel, RT-310, Teverelix trifluoroacetate, Fexapotide, GI198745, DKF-313, Silodosin, ASP4901, Tadalafil, PRX302, Levitra (Vardenafil, BAY38-9456), PRX302, WC3055, NX-1207, QLT0074, Tamsulosin Hydrochloride, Ningmitai capsule, and others are expected to have a significant impact on the Benign Prostatic Hyperplasia market in the coming years.
*
In October 2023, Nymox Pharmaceutical Corporation has disclosed that their recent application for Fexapotide Triflutate, intended for addressing symptoms associated with benign prostate enlargement (commonly known as benign prostatic hyperplasia or BPH), has been officially recognized by the UK authorities, specifically the UK Medicines & Healthcare products Regulatory Agency (MHRA). The formal evaluation process is now underway. The product will be marketed under the trademarked name NYMOZARFEX. The Marketing Authorization Application (MAA) was lodged on September 25, 2023.
Benign Prostatic Hyperplasia Overview
Benign prostatic hyperplasia (BPH), also known as prostate gland enlargement, is a common condition that affects older men. The prostate gland, which is part of the male reproductive system, surrounds the urethra (the tube that carries urine from the bladder out of the body).
Get a Free Sample PDF Report to know more about Benign Prostatic Hyperplasia Pipeline Therapeutic Assessment-
https://www.delveinsight.com/report-store/benign-prostatic-hyperplasia-bph-pipeline-insight [https://www.delveinsight.com/report-store/benign-prostatic-hyperplasia-bph-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=gpr]
Emerging Benign Prostatic Hyperplasia Drugs Under Different Phases of Clinical Development Include:
*
AIV007: AiViva BioPharma
*
CKD-846: Chong Kun Dang
*
DTT106: EMS
*
Paclitaxel: Urotronic Inc.
*
RT-310: Resurge Therapeutics Inc
*
Teverelix trifluoroacetate: Antev
*
Fexapotide: Nymox Pharmaceutical
*
GI198745: GlaxoSmithKline
*
DKF-313: Dongkook Pharmaceutical
*
Silodosin: RECORDATI GROUP
*
ASP4901: Astellas Pharma Inc
*
Tadalafil: Eli Lilly and Company
*
PRX302: Sophiris Bio Corp
*
Levitra (Vardenafil, BAY38-9456): Bayer
*
PRX302: Sophiris Bio Corp
*
WC3055: Warner Chilcott
*
NX-1207: Nymox Corporation
*
QLT0074: QLT Inc.
*
Tamsulosin Hydrochloride: NeoTract, Inc.
*
Ningmitai capsule: Xintian Pharmaceutical
Benign Prostatic Hyperplasia Route of Administration
Benign Prostatic Hyperplasia pipeline report provides the therapeutic assessment of the pipeline drugs by the Route of Administration. Products have been categorized under various ROAs, such as
*
Oral
*
Parenteral
*
Intravenous
*
Subcutaneous
*
Topical
Benign Prostatic Hyperplasia Molecule Type
Benign Prostatic Hyperplasia Products have been categorized under various Molecule types, such as
*
Monoclonal Antibody
*
Peptides
*
Polymer
*
Small molecule
*
Gene therapy
Benign Prostatic Hyperplasia Pipeline Therapeutics Assessment
*
Benign Prostatic Hyperplasia Assessment by Product Type
*
Benign Prostatic Hyperplasia By Stage and Product Type
*
Benign Prostatic Hyperplasia Assessment by Route of Administration
*
Benign Prostatic Hyperplasia By Stage and Route of Administration
*
Benign Prostatic Hyperplasia Assessment by Molecule Type
*
Benign Prostatic Hyperplasia by Stage and Molecule Type
DelveInsight's Benign Prostatic Hyperplasia Report covers around 5+ products under different phases of clinical development like
*
Late-stage products (Phase III)
*
Mid-stage products (Phase II)
*
Early-stage product (Phase I)
*
Pre-clinical and Discovery stage candidates
*
Discontinued & Inactive candidates
*
Route of Administration
Further Benign Prostatic Hyperplasia product details are provided in the report. Download the Benign Prostatic Hyperplasia pipeline report to learn more about the emerging Benign Prostatic Hyperplasia therapies [https://www.delveinsight.com/sample-request/benign-prostatic-hyperplasia-bph-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=gpr]
Some of the key companies in the Benign Prostatic Hyperplasia Therapeutics Market include:
Key companies developing therapies for Benign Prostatic Hyperplasia are - Eli Lilly & Company, Boehringer Ingelheim, Merck & Co. Inc., Allergan PLC, Astellas Pharma Inc., and others.
Benign Prostatic Hyperplasia Pipeline Analysis:
The Benign Prostatic Hyperplasia pipeline report provides insights into
*
The report provides detailed insights about companies that are developing therapies for the treatment of Benign Prostatic Hyperplasia with aggregate therapies developed by each company for the same.
*
It accesses the Different therapeutic candidates segmented into early-stage, mid-stage, and late-stage of development for Benign Prostatic Hyperplasia Treatment.
*
Benign Prostatic Hyperplasia key companies are involved in targeted therapeutics development with respective active and inactive (dormant or discontinued) projects.
*
Benign Prostatic Hyperplasia Drugs under development based on the stage of development, route of administration, target receptor, monotherapy or combination therapy, a different mechanism of action, and molecular type.
*
Detailed analysis of collaborations (company-company collaborations and company-academia collaborations), licensing agreement and financing details for future advancement of the Benign Prostatic Hyperplasia market.
The report is built using data and information traced from the researcher's proprietary databases, company/university websites, clinical trial registries, conferences, SEC filings, investor presentations, and featured press releases from company/university websites and industry-specific third-party sources, etc.
Download Sample PDF Report to know more about Benign Prostatic Hyperplasia drugs and therapies [https://www.delveinsight.com/sample-request/benign-prostatic-hyperplasia-bph-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=gpr]
Benign Prostatic Hyperplasia Pipeline Market Drivers
*
The prevalence of this disease drives demand for treatments, creating a growth opportunity for pharmaceutical companies and medical device manufacturers in the BPH market thus fuels research, development, and innovation, leading to the introduction of new therapies and technologies.
Benign Prostatic Hyperplasia Pipeline Market Barriers
*
The presence of significant side effects linked to BPH treatments can deter patient adherence and acceptance, limiting market expansion as concerns about safety and tolerability affect physician prescribing patterns and hinder uptake of therapies
Scope of Benign Prostatic Hyperplasia Pipeline Drug Insight
*
Coverage: Global
*
Key Benign Prostatic Hyperplasia Companies: AiViva BioPharma, Chong Kun Dang, EMS, Urotronic Inc., Resurge Therapeutics Inc, Antev, Nymox Pharmaceutical, GlaxoSmithKline, Dongkook Pharmaceutical, RECORDATI GROUP, Astellas Pharma Inc, Eli Lilly and Company, Sophiris Bio Corp, Bayer, Sophiris Bio Corp, Warner Chilcott, Nymox Corporation, QLT Inc., NeoTract, Inc., Xintian Pharmaceutical, and others
*
Key Benign Prostatic Hyperplasia Therapies: AIV007, CKD-846, DTT106, Paclitaxel, RT-310, Teverelix trifluoroacetate, Fexapotide, GI198745, DKF-313, Silodosin, ASP4901, Tadalafil, PRX302, Levitra (Vardenafil, BAY38-9456), PRX302, WC3055, NX-1207, QLT0074, Tamsulosin Hydrochloride, Ningmitai capsule, and others
*
Benign Prostatic Hyperplasia Therapeutic Assessment: Benign Prostatic Hyperplasia current marketed and Benign Prostatic Hyperplasia emerging therapies
*
Benign Prostatic Hyperplasia Market Dynamics: Benign Prostatic Hyperplasia market drivers and Benign Prostatic Hyperplasia market barriers
Request for Sample PDF Report for Benign Prostatic Hyperplasia Pipeline Assessment and clinical trials [https://www.delveinsight.com/sample-request/benign-prostatic-hyperplasia-bph-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=gpr]
Table of Contents
1. Benign Prostatic Hyperplasia Report Introduction
2. Benign Prostatic Hyperplasia Executive Summary
3. Benign Prostatic Hyperplasia Overview
4. Benign Prostatic Hyperplasia- Analytical Perspective In-depth Commercial Assessment
5. Benign Prostatic Hyperplasia Pipeline Therapeutics
6. Benign Prostatic Hyperplasia Late Stage Products (Phase II/III)
7. Benign Prostatic Hyperplasia Mid Stage Products (Phase II)
8. Benign Prostatic Hyperplasia Early Stage Products (Phase I)
9. Benign Prostatic Hyperplasia Preclinical Stage Products
10. Benign Prostatic Hyperplasia Therapeutics Assessment
11. Benign Prostatic Hyperplasia Inactive Products
12. Company-University Collaborations (Licensing/Partnering) Analysis
13. Benign Prostatic Hyperplasia Key Companies
14. Benign Prostatic Hyperplasia Key Products
15. Benign Prostatic Hyperplasia Unmet Needs
16 . Benign Prostatic Hyperplasia Market Drivers and Barriers
17. Benign Prostatic Hyperplasia Future Perspectives and Conclusion
18. Benign Prostatic Hyperplasia Analyst Views
19. Appendix
20. About DelveInsight
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences. It supports Pharma companies by providing comprehensive end-to-end solutions to improve their performance. It also offers Healthcare Consulting Services, which benefits in market analysis to accelerate business growth and overcome challenges with a practical approach.
Media Contact
Company Name: DelveInsight
Contact Person: Gaurav Bora
Email:Send Email [https://www.abnewswire.com/email_contact_us.php?pr=benign-prostatic-hyperplasia-pipeline-drugs-analysis-report-2024-updates-fda-approvals-clinical-trials-therapies-moa-roa-by-delveinsight-boehringer-ingelheim-merck-co-inc-allergan-plc]
Phone: +14699457679
Address:304 S. Jones Blvd #2432
City: Las Vegas
State: NV
Country: United States
Website: https://www.delveinsight.com/
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Benign Prostatic Hyperplasia Pipeline Drugs Analysis Report, 2024 Updates: FDA Approvals, Clinical Trials, Therapies, MOA, ROA by DelveInsight | Boehringer Ingelheim, Merck & Co. Inc., Allergan PLC here
News-ID: 3485935 • Views: …
More Releases from ABNewswire

Real-time Location Systems Market worth $16.2 billion by 2028, at a CAGR of 25.5 …
The global real-time location systems market in terms of revenue was estimated to be worth $5.2 billion in 2023 and is poised to reach $16.2 billion by 2028, growing at a CAGR of 25.5% during the forecast period.
The report "Real-time Location Systems Market [https://www.marketsandmarkets.com/Market-Reports/real-time-location-systems-market-1322.html] by Hardware (Tags/Badges, Readers/Trackers), Technology (RFID, Wi-Fi, UWB, BLE, Infrared, Ultrasound, GPS, Zigbee), Application (Inventory/Asset Tracking, Personnel Monitoring), Vertical, Region - Global Forecast to 2028" The…
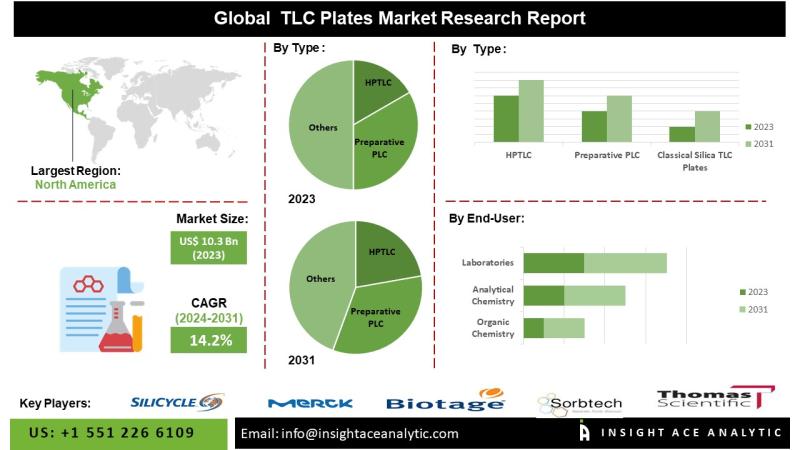
TLC Plates Market Poised for Growth: $29.5 Million Opportunity by 2031
TLC Plates Market is valued at US$ 10.3 Bn in 2023, and it is expected to reach US$ 29.5 Bn by 2031, with a CAGR of 14.2% during the forecast period of 2024-2031.
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global TLC Plates Market - (By Type (HPTLC, Preparative PLC, Classical Silica TLC Plates), By Application (Organic chemistry, Analytical chemistry, Laboratories)), Trends, Industry Competition…
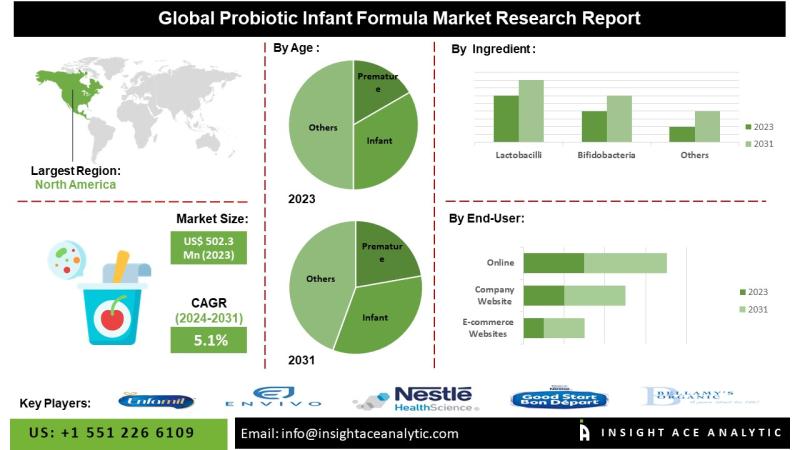
Probiotic Infant Formula Market Heats Up: Parents Seek Digestive and Immune Supp …
Probiotic Infant Formula Market is valued at US$ 502.3 Mn in 2023, and it is expected to reach US$ 739.4 Mn by 2031, with a CAGR of 5.1% during the forecast period of 2024-2031.
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the " [https://www.insightaceanalytic.com/report/probiotic-infant-formula-market/2479]- (By Age (Premature, Infant and Toddler), By Ingredient (Lactobacilli, Bifidobacteria and Others), By Sales Channel (Online and Offline), By Region, Trends,…

Eldercare-Assistive Robots Market: Expected to Reach $5.98 Billion by 2031
Eldercare-Assistive Robots Market is valued at US$ 2.5 Bn in 2023, and it is expected to reach US$ 5.9 Bn by 2031, with a CAGR of 11.7% during the forecast period of 2024-2031.
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the " [https://www.insightaceanalytic.com/report/eldercare-assistive-robots-market/2469] - (By Type (Physically Assistive Robots, Socially Assistive Robots), Trends, Industry Competition Analysis, Revenue and Forecast To 2031."
According to the latest research…
More Releases for Benign
Benign Prostatic Hyperplasia Therapeutics Market Clinical Survey Report 2020
The Benign Prostatic Hyperplasia Therapeutics Market report is a compilation of first-hand information, qualitative and quantitative assessment by industry analysts, inputs from industry experts and industry participants across the value chain. The report provides in-depth analysis of parent market trends, macro-economic indicators and governing factors along with market attractiveness as per segments. The report also maps the qualitative impact of various market factors on market segments and geographies.
Click the link…
Benign prostatic hyperplasia: BPH drugs versus Dr Allen’s Device
The Global Treatment Market of Benign prostatic hyperplasia (BPH) was valued at $10,688.72 million in 2017 and is projected to reach $20,096.68 million by 2025 at a CAGR of 8.1% from 2018 to 2025.
Thermobalancing therapy with Dr Allen’s Device was patented by the United States Patent and Trademark Office. Over a decade, thousands of men with BPH used or are using this non-invasive and side-effects free Dr Allen’s Device.…
Cold Plasma Market Benign Rate by 2025
Global Cold Plasma Market: Snapshot
Cold plasma, additionally called non-warm plasma or non-balance plasma, is a sort of plasma—ordinarily known as the fourth condition of issue—which has gas particles show at direct temperature and electrons at moderately high temperatures. The properties of plasma have been widely looked into in the course of recent decades and an extensive number of front line utilizations of cold plasma innovation has exuded.
Cold plasma isn't in…
Benign Prostatic Hyperplasia Therapeutics Market Epidemiology Analysis, Therapy, …
The global benign prostatic hyperplasia therapeutics market is expected to witness significant growth, as increased number of effective treatments are available for cancer. Increasing awareness regarding various cancer treatment drugs, technological advancements, and growing demand for safe and effective medications are acting as the major growth drivers for the benign prostatic hyperplasia therapeutics market. Globally, regulatory bodies are adding to the growth of the market with provision of funding, grants…
Benign Prostatic Hyperplasia and Prostatitis Devicebased Treatments Market
ReportsWorldwide has announced the addition of a new report title Benign Prostatic Hyperplasia and Prostatitis Devicebased Treatments Market to its growing collection of premium market research reports.
This analysis includes a discussion of products, competitors, market forecasts, and opportunities in the global market for BPH and prostatitis treatment devices.
Topics Covered Include:
An overview of device-based and pharmacological therapies for BPH and prostatitis.
Global and country/region-specific market forecasts, including forecasts by product segment.
Competitive analyses…
Benign Prostatic Hyperplasia Therapeutics Development Pipeline Overview for H1 2 …
2017 Benign Prostatic Hyperplasia pipeline market report of on global industry analysis and opportunity assessment H1 report provides comprehensive information on the therapeutics under development for Benign Prostatic Hyperplasia (Male Health), complete with analysis by stage of development, drug target, mechanism of action (MoA), route of administration (RoA) and molecule type.
Get Discount on this Research Report at http://www.reportsnreports.com/contacts/discount.aspx?name=1010746
Benign Prostatic Hyperplasia (BPH) is a non-cancerous enlargement of the prostate, a…
