Press release
Pharmacovigilance Market 2024-2031 | Exclusive Study Report
Pharmacovigilance Market to reach over USD 19.50 Bn by the year 2031 - Exclusive Report by InsightAce Analytic"Pharmacovigilance Market" in terms of revenue was estimated to be worth $8.76 billion in 2023 and is poised to reach $19.50 billion by 2031, growing at a CAGR of 10.77% from 2024 to 2031 according to a new report by InsightAce Analytic.
Get Free Sample Report @ https://www.insightaceanalytic.com/request-sample/1608
Latest Drivers Restraint and Opportunities Market Snapshot:
Key factors influencing the global Pharmacovigilance Market are:
• Increasing regulatory requirements
• Growing innovative pharmaceuticals
• Rising interest in tailored healthcare
The following are the primary obstacles to the Pharmacovigilance Market 's expansion:
• Standards for the safe administration of medications
• Concerns about data security
• Distressed healthcare workers
Future expansion opportunities for the global Pharmacovigilance Market include:
• More and more novel medications
• Rising tide of outsourcing
• Accelerating research and development efforts
Market Analysis:
The market is expected to grow rapidly because of the increasing number of pharmaceuticals used by patients and their increasing expectations regarding the safety of these medications. Outsourcing pharmacovigilance to a company that offers superior software and services might lead to more income and overall company growth.
List of Prominent Players in the Pharmacovigilance Market:
• Cognizant
• IBM Corporation
• ICON plc
• Pharmaceutical Development Group, Inc.
• Labcorp Drug Development
• ArisGlobal
• United BioSource LLC
Pharmacovigilance Market Report Scope:
Report Attribute Specifications
Market size value in 2023 USD 8.76 billion
Revenue forecast in 2031 USD 19.50 billion
Growth rate CAGR CAGR of 10.77% from 2024 to 2031
Quantitative units Representation of revenue in US$ Bn, and CAGR from 2024 to 2031
Historic Year 2019 to 2023
Forecast Year 2024-2031
Report coverage The forecast of revenue, the position of the company, the competitive market structure, growth prospects, and trends
Segments covered By Service Provider, Product Life Cycle, Type, Process Flow, Therapeutic Area, And End-Use
Regional scope North America; Europe; Asia Pacific; Latin America; Middle East & Africa
Recent Developments:
• In January 2024, Cognizant and Microsoft released the Innovation Assistant, an AI-powered tool that will enhance Bluebolt, Cognizant's internal innovation initiative. The tool is built on Microsoft Azure OpenAI Service and uses generative algorithms.
• In January 2024, ICON plc, a frontrunner in healthcare intelligence and clinical research, released a whitepaper titled "Optimising biotech funding. The present scenario and potential R&D tactics biotech companies might implement to entice and make the most of investment capital are discussed here.
• In November 2023, IBM announced that it had inked three MoUs with three entities linked to the Department of Computers and the Internet (MeitY) to support and accelerate innovation in quantum technology, AI, and semiconductors for the benefit of the Indian government.
Curious about this latest version of the report? @ https://www.insightaceanalytic.com/enquiry-before-buying/1608
Pharmacovigilance Market Dynamics:
Market Drivers: Increasing Regulatory Requirements
The increasing regulatory requirements on clinical trials propel the drug safety solutions and pharmacovigilance industry. To ensure patient safety and the precision of the data collected from clinical trials, regulatory agencies worldwide have imposed more stringent regulations and rules. These regulations stress the importance of pharmacovigilance procedures and strong drug safety solutions for efficiently monitoring and reporting adverse events in clinical trials. The increasing investment in comprehensive pharmacovigilance systems and solutions by research institutes and pharmaceutical corporations to meet regulatory obligations is driving the expansion of drug safety solutions and pharmacovigilance.
Challenges: Standards for the Safe Administration of Medications
The pharmaceutical sector is subject to strict regulations governing its operations, including reporting and monitoring adverse drug reactions. The allocation of resources, infrastructure, and expertise are all areas where businesses struggle to comply with these intricate requirements. The resources required to adhere to regulatory requirements could make adopting and implementing pharmacovigilance systems and medication safety solutions challenging. One major obstacle to the expansion of this business is the difficulty of understanding and complying with the many different international regulations.
North America is Expected to Grow with the Highest CAGR During the Forecast Period
The North America Pharmacovigilance Market is likely to register a significant revenue share and develop at a rapid CAGR in the near future. This is because of things like a dramatic increase in drug overdoses, a plethora of negative drug reactions, and massive money spent on drug research by big pharma. Strict reporting systems and steps to ensure the safety of drugs have been in high demand in the area for these reasons. The massive output of pharmaceuticals has boosted clinical trials and post-marketing monitoring demands, which have helped spur growth in the area's marketplace.
Segmentation of Pharmacovigilance Market-
By Service Provider-
• In-house
• Contract outsourcing
By Product Life Cycle-
• Pre-clinical
• Phase I
• Phase II
• Phase III
• Phase IV
By Type -
• Spontaneous Reporting
• Intensified ADR Reporting
• Targeted Spontaneous Reporting
• Cohort Event Monitoring
• EHR Mining
By Process Flow-
• Case Data Management
o Case Logging
o Case Data Analysis
o Medical Reviewing & Reporting
• Signal Detection
o Adverse Event Logging
o Adverse Event Analysis
o Adverse Event Review & Reporting
• Risk Management System
o Risk Evaluation System
o Risk Mitigation System
By Therapeutic Area-
• Oncology
• Neurology
• Cardiology
• Respiratory Systems
• Others
By End Use
• Pharmaceuticals
• Biotechnology Companies
• Medical Device Manufacturers
• Others
By Region-
North America-
• The US
• Canada
• Mexico
Europe-
• Germany
• The UK
• France
• Italy
• Spain
• Rest of Europe
Asia-Pacific-
• China
• Japan
• India
• South Korea
• Southeast Asia
• Rest of Asia Pacific
Latin America-
• Brazil
• Argentina
• Rest of Latin America
Middle East & Africa-
• GCC Countries
• South Africa
• Rest of Middle East and Africa
For More Customization @ https://www.insightaceanalytic.com/customisation/1608
Contact Us:
InsightAce Analytic Pvt. Ltd.
Tel.: +1 718 593 4405
Email: info@insightaceanalytic.com
Site Visit: www.insightaceanalytic.com
Follow Us on LinkedIn @ bit.ly/2tBXsgS
Follow Us On Facebook @ bit.ly/2H9jnDZ
About Us:
InsightAce Analytic is a market research and consulting firm that enables clients to make strategic decisions.
Our qualitative and quantitative market intelligence solutions inform the need for market and competitive intelligence to expand businesses.
We help clients gain a competitive advantage by identifying untapped markets, exploring new and competing technologies, segmenting potential markets, and repositioning products.
Our expertise is in providing syndicated and custom market intelligence reports with an in-depth analysis with key market insights in a timely and cost-effective manner.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Pharmacovigilance Market 2024-2031 | Exclusive Study Report here
News-ID: 3418286 • Views: …
More Releases from InsightAce Analytic Pvt.Ltd
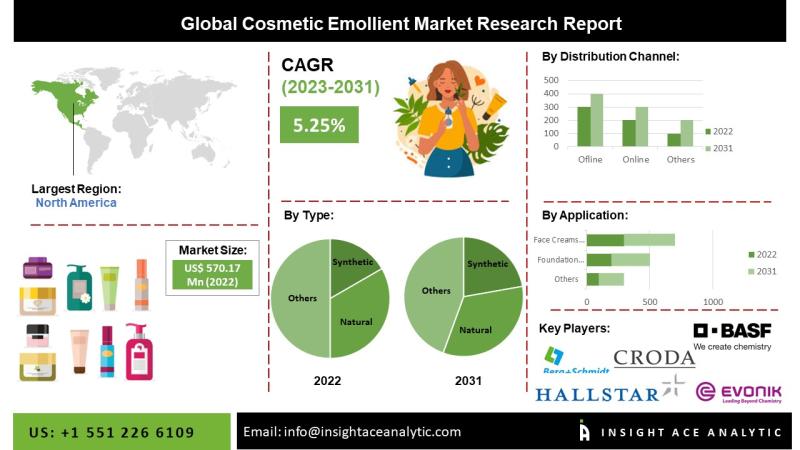
Cosmetic Emollient Market Future Scope and Latest Trends Analysis Report
Cosmetic Emollient Market
Cosmetic Emollient Market to reach over USD 895.49 million by the year 2031 - Exclusive Report by InsightAce Analytic
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Cosmetic Emollient Market Size, Share & Trends Analysis Report By Application (Foundation Creams, Face Creams & Moisturizers, Body Creams & Lotions, Sunscreens, and Others), Type (Synthetic and Natural), Distribution Channel (Offline and Online) - Market…
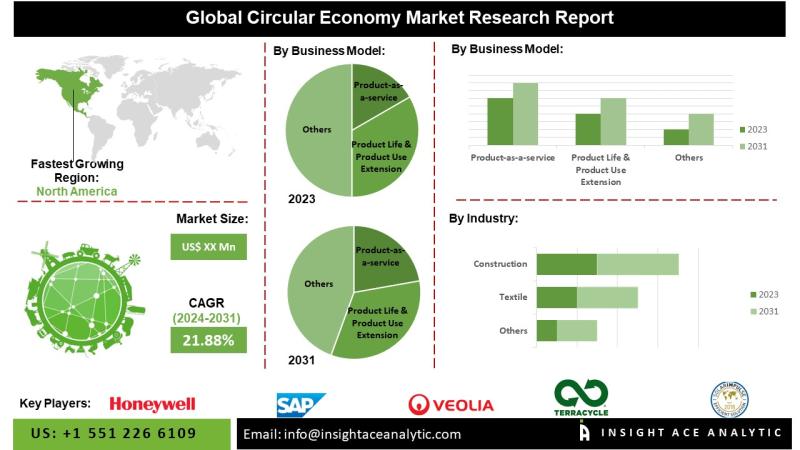
Circular Economy Market Latest Report with Forecast to 2031
Circular Economy Market to Record an Exponential CAGR by 2031 - Exclusive Report by InsightAce Analytic Pvt. Ltd.
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Circular Economy Market- (By Business Model (Product-as-a-service (Leasing, Sharing, and Subscription Models), Product Life & Product Use Extension (Reusing/Reselling, Repairing, and Remanufacturing), and Resource Recovering/Upcycling), By Industry (Fashion & Textile Industry, Consumer Electronics Industry, Construction Industry, Automotive Industry,…
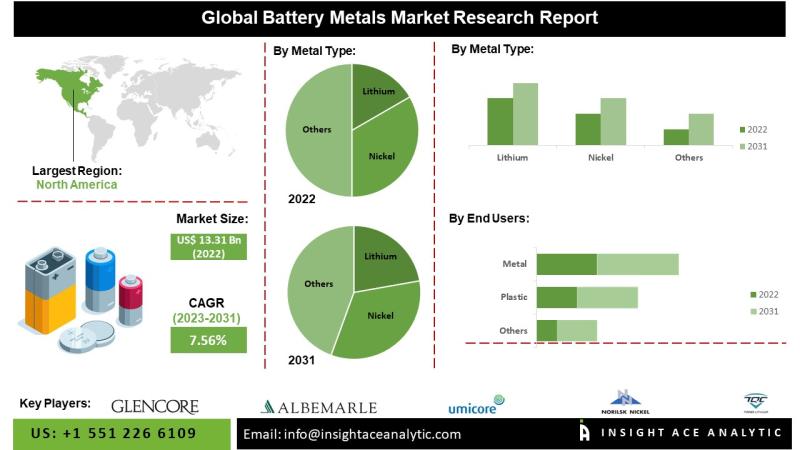
Battery Metals Market Reviews Analysis Report 2024
Battery Metals Market worth $25.32 Mn by 2031 - Exclusive Report by InsightAce Analytic Pvt. Ltd.
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Battery Metals Market - (By End-Users (Transportation, Consumer Electronics, Energy Storage Systems, Others), By Metal Type (Lithium, Nickel, Cobalt, Others)), Trends, Industry Competition Analysis, Revenue and Forecast To 2031."
According to the latest research by InsightAce Analytic, the Global Battery Metals…
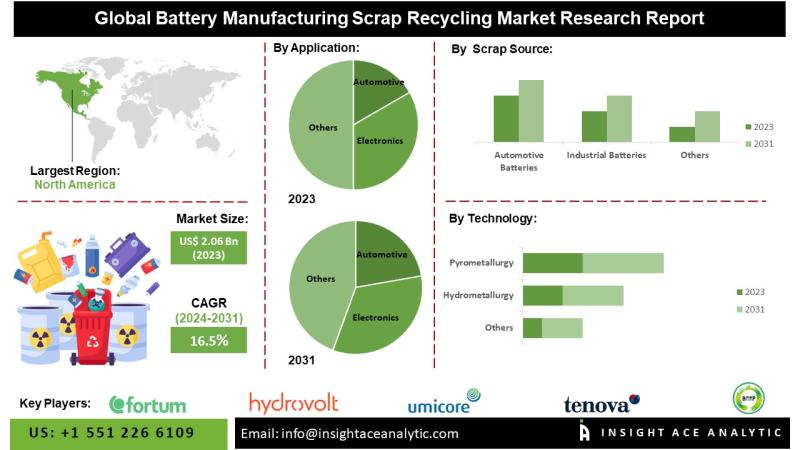
Battery Manufacturing Scrp Recycling Market - Recent Innovations and Upcoming Tr …
Battery Manufacturing Recycling Market worth $6.92 Mn by 2031 - Exclusive Report by InsightAce Analytic Pvt. Ltd.
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Battery Manufacturing Recycling Market - (By Application (Automotive, Electronics, Energy and Power, Aerospace and Defense, Construction, Others), By Source (Automotive Batteries, Industrial Batteries, Consumer Electronics Batteries, Others), By Technology (Hydrometallurgy, Pyrometallurgy, Others)), Trends, Industry Competition Analysis, Revenue and…
More Releases for Pharmacovigilance
Pharmacovigilance Outsourcing Market 2021 | Detailed Report
Pharmacovigilance Outsourcing Market Forecasts report provided to identify significant trends, drivers, influence factors in global and regions, agreements, new product launches and acquisitions, Analysis, market drivers, opportunities and challenges, risks in the market, cost and forecasts to 2027.
Get Free Sample PDF (including full TOC, Tables and Figures) of Pharmacovigilance Outsourcing Market @ https://www.reportsnreports.com/contacts/requestsample.aspx?name=4964765
The report provides a comprehensive analysis of company profiles listed below:
- Clinquest Group B.V
- Cognizant
- LabCorp
- IBM
-…
Global Pharmacovigilance Market & Forecasts
According to a new market research report published by Global Market Estimates, the Global Pharmacovigilance Market is expected to grow at a CAGR of 13.55% during the forecast period. The demand for pharmacovigilance is projected to be high due to the increasing demand for pharmacovigilance services and rising cost-effective initiatives for SMEs companies.
Browse 145 Market Data Tables and 118 Figures spread through 168 Pages and in-depth TOC on "Pharmacovigilance Market…
Pharmacovigilance and Drug Safety Software Market: Pharmacovigilance and Drug Sa …
The global pharmacovigilance and drug safety software market is projected to create new opportunities in the coming years on account of the escalating complexity of drug safety regulations. Among the key contributing factors of the market could be tighter regulatory inspections implemented to control the rise of the incidences of drug-associated adverse events and spiraling concerns pertaining to patient safety.
Pharmaceutical and biotechnology companies are predicted to largely adopt pharmacovigilance software…
Rising Number of Government Pharmacovigilance Centers to Boost Pharmacovigilance …
Pharmacovigilance is a key component of an effective drug regulation system for monitoring and evaluating adverse drug reactions (ADRs). Pharmacovigilance activities are an important part of clinical research and are growing at a significant pace. At present, the global network of pharmacovigilance centers, harmonized by Uppsala Monitoring Centre, are operating on the global level for appropriate functioning of the process of drug safety monitoring across the world. Large volume of…
Pharmacovigilance Market | Forecast up to 2020
Pharmacovigilance is a key component of an effective drug regulation system for monitoring and evaluating adverse drug reactions (ADRs). Pharmacovigilance activities are an important part of clinical research and are growing at a significant pace. At present, the global network of pharmacovigilance centers, harmonized by Uppsala Monitoring Centre, are operating on the global level for appropriate functioning of the process of drug safety monitoring across the world. Large volume of…
Pharmacovigilance Market Research Report Forecast to 2020
Pharmacovigilance is a crucial component of an effective drug regulation system and is used for evaluating and monitoring adverse drug reactions. Pharmacovigilance activities are essential for conducting clinical research. Currently, the global network of pharmacovigilance centers is operating effectively to assure appropriate functioning of drug safety procedures. The large volume of international adverse drug reaction reports collected and stored in a central database assists the national drug regulatory authorities to…
