Press release
curasan AG launches bone substitute Osbone® – the synthetic alternative without biological history
Frankfurt/Kleinostheim, Germany, 16th March 2010 – In Germany alone, every year about 800,000 tooth implants are placed. In the case of almost every second patient bone augmentation is necessary, because the jawbone atrophy is already too advanced. The development of bone substitute materials has advanced considerably over the last few years, particularly in the area of scaffold substances. There is a broad spectrum ranging from autologous, fully synthetic to xenogenic materials. “With Osbone® we now have a next generation of fully synthetic hydroxyapatite that, in contrast to bovine materials, involves absolutely no potential infection or allergy risks, which cannot ever be completely discounted with xenogenic materials”, explains Dr. Wolf-Dietrich Hübner, Medical Director at curasan AG.The earlier generation of synthetic hydroxyapatites did not always exhibit the desired clinical results, due to their low porosity and insufficient biocompatibility. Osbone®, on the other hand, is extremely biocompatible due to its high porosity and modern method of manufacture, and enables rapid osseointegration and therefore a stable implant bedding for subsequent implantation. Synthetically-produced drugs and medical devices can be manufactured to higher levels of purity and will consequently fulfil the requirements of a progressive medical science. “Until now, there were few practical alternatives to bovine substitute materials and the use of autologous bone substances. Here, the new synthetic materials are very promising, also because it clearly simplifies the informing of patients and reduces the risk of liability”, confirms Dr. Andreas Holweg, specialist for oral maxillofacial surgery from Fulda.
Indications and optimum application
Osbone® is a synthetic hydroxyapatite and, by virtue of its properties, is suitable for application in the field of oral and maxillofacial surgery for many indications.
Commensurate with its slow resorption kinetics and its irregular polygonal morphology, Osbone® is particularly suitable for filling defects or for the augmentation of areas where a stable bedding is required for implants. In order to achieve optimum results, all bone fragments, necrotic tissue and connective tissue should be carefully removed to prepare the implant bedding. Direct contact of Osbone® with the bleeding, vital bone and a thorough debriding of the bone prior to introduction, support the colonisation with osteocytes and blood vessel infiltration. Incidentally, this advice also applies in equal measure to other bone substitute materials. Before being inserted into the defect, Osbone® should be mixed with autologous blood from the damaged region, as the body has already enriched this area with factors which participate in wound healing. Contained within the viscous consistency of the coagulating, autologous blood, Osbone® may be easily applied to the defect. Use in conjunction with autologous spongiosa, bone marrow aspirate or platelet-rich plasma (PRP) is also possible. The multiporous structure of the granulate forms the basis for the development of the material and for access of cell nutrients.
In addition
Osbone® makes it easier for doctors to inform patients. Due to its synthetic manufacture, explanations about the biological origins of the material, its source and potential residual risks of infection and allergic reaction induced by the product no longer apply. The physician is in a position to offer his or her patients an alternative to bovine hydroxyapatite. The doctor can thereby fulfil the legally prescribed duty to inform patients without any worries. Furthermore he can meet the requirements of the patient’s right to self-determination.
Additional information available at www.osbone.de
About curasan AG
curasan AG develops, produces and markets products from the future-oriented field of regenerative medicine. It finds application in bone and tissue regeneration, tissue culture, wound healing and arthrosis, and aims at orthopaedic, trauma and spine surgeons, specialists in sports medicine as well, as implantologists, oral and maxillofacial surgeons and dentists involved in dental surgery.
Press Contact:
curasan AG
Andrea Weidner
Lindigstrasse 4
63801 Kleinostheim
Germany
Tel. +49 (0)6027 40 900-51
Fax +49 (0)6027 40 900-39
andrea.weidner@curasan.de
www.curasan.de
fr financial relations gmbh
Jörn Gleisner
Tel. +49 (0)69 95 90 83 20
Fax +49 (0)69 95 90 83 99
j.gleisner@finanical-relations.de
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release curasan AG launches bone substitute Osbone® – the synthetic alternative without biological history here
News-ID: 123893 • Views: …
More Releases from curasan AG
curasan AG: Dental Business Unit acquired as of June 1
Kleinostheim, Germany, June 6, 2013 – curasan AG, Germany, has acquired a large part of the “Oral Regenerative Medicine” division of Riemser Pharma GmbH, Germany, effective June 1, 2013.
curasan AG now owns the marketing and distribution rights for the products covered by the contract and is assuming the sales structures, including the na-tional and international customer base.
As a result of this change, curasan has immediate access to the dental market…
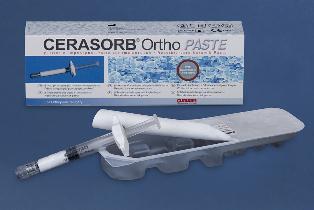
curasan AG now sells bone regeneration material in injectable form
Kleinostheim, 05.12.2012 – curasan AG, listed on the General Standard (ISIN: DE 000 549 453 8), has introduced its new bone regeneration material CERASORB® Ortho Paste to the German and European markets at the beginning of December.
CERASORB® Ortho Paste is comprised of fine granules of the proven, fully resorbable, synthetic material CERASORB® M Ortho suspended in a hyaluronic acid hydrogel-matrix. The paste-like product has the advantage that it is…
curasan AG commences operations in the European market with the appointment of a …
Kleinostheim, 15.02.2012 – curasan AG, listed in the General Standard (ISIN: DE 000 549 453 8), created a new post on 1st February 2012 in relation to the active expansion of its European sales network in the fields of Orthopaedics and trauma surgery. Mr. Lee McCann BSc Business Administration (North West University of Applied Science, London, U.K.), will be operating as the organisation’s International Sales Director for Orthobiologics with immediate…
curasan AG: Novel bone regeneration material approved for sale in Europe
Kleinostheim, Germany – curasan AG, which is listed in the General Standard of the German Stock Exchange (ISIN: DE 000 549 453 8), has received European approval for a novel synthetic bone regeneration material.
Osseolive® is a bioactive, poly-crystalline calcium-alkali-phosphate ceramic, which has a stimulating effect on bone formation as well as on bone mineralisation.
“With Osseolive® emerging from our well-stocked development pipeline, we now have an ideal extension to our tricalcium-phosphate…
More Releases for Osbone®
Ultimate Chemicals® Featured in Manufacturing Marvels® on The Fox Business Net …
MOORE, OK., Oct. 8, 2021/ -- Ultimate Chemicals® Inc., an integrated manufacturer and supplier of chemicals and related services, is pleased to announce it will be showcased in a broadcast of Manufacturing Marvels® scheduled to air on October 14, 2021 at approximately 9:30-9:44 p.m. EST on The Fox Business Network® (FBN).
Ultimate Chemicals has created their products to help restore surfaces to their optimum surface capability. Non-destructive to the surfaces to…
Sunera Technologies, Inc Joins SAP® PartnerEdge® Delivering SAP® S/4HANA
Sunera Technologies, Inc Joins SAP® PartnerEdge® Delivering SAP® S/4HANA and Intelligent Enterprise to Manufacturing, Consumer Products, Higher education, and research, Industrial machinery and components, Life sciences and Automotive Industries
Chicago, IL, June 10, 2020 – Sunera Technologies, Inc announced that it had joined the SAP® PartnerEdge® program as a Re-sell & Services partner, through which it will resell SAP solutions to midsize organizations in North America. Through this engagement, SuneraTech…
Infosim® announces release of StableNet® 7.5
Würzburg/Austin/Singapore, October 26th, 2015 – Infosim®, the technology leader in automated Service Fulfillment and Service Assurance solutions, today announced the release of version 7.5 of its award-winning software suite StableNet® for Telco and Enterprise customers.
StableNet® 7.5 provides a significant number of powerful new features, including:
• Dynamic Rule Generation (DRG); a new and revolutionary Fault Management concept
• REST interface supporting the new StableNet® iPhone (and upcoming Android) app
• Highly customizable dashboard in both the…
curasan AG receives license for Osbone® Dental in the USA
Kleinostheim, Germany, 10th February 2011 - curasan AG, which is listed in the General Standard of the German Stock Exchange (ISIN: DE 000 549 453 8), has received FDA certification and, therefore, marketing authorization throughout the USA of the synthetic bone replacement material Osbone® Dental which already has been authorized in the EU as Osbone®.
Osbone® Dental is suitable for utilisation in general bone surgery, particularly in implantology and oral…
American Megatrends Announces AMIBIOS®8 & Aptio®4.x Support for the Intel® Xe …
ATLANTA, GEORGIA - American Megatrends (AMI) is pleased to announce that its AMIBIOS®8 & Aptio®4.x products feature support for the new Intel® Xeon® 5500 Platform.
The Intel® Xeon® 5500 platform combines the Intel® Xeon® Processor 5500 Series with the Intel® 5520 Chipset for embedded, storage, security and communications infrastructure applications in a wide range of form factors. There are four different processor options for this platform with seven year lifecycle support…
CNUX .NET Providers for DB2® Earns IBM® DB2® Data Server Certification
CNUX Technologies Inc. announced today that CNUX .NET Providers for DB2® - Standard and Web Service Edition have achieved "Ready for IBM DB2 Data Server Software" status. With this designation CNUX customers can be assured that CNUX .NET Providers interacts and operates seamlessly with IBM® DB2® software.
The "Ready for IBM DB2 Data Server Software" program is a process designed for independent software vendors (ISVs) and solution integrators to validate their…