Press release
Clinical Trial Investigative Site Network Market Report Explores Reviews Analysis Report 2024
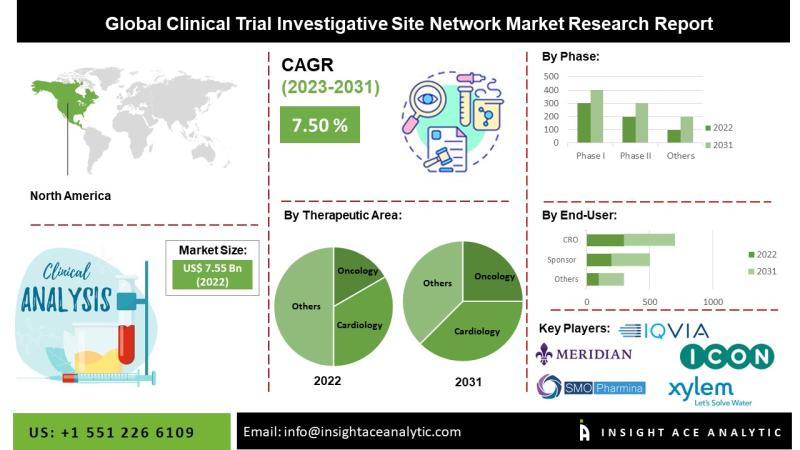
Clinical Trial Investigative Site Network Market" in terms of revenue was estimated to be worth $7.99 billion in 2023 and is poised to reach $14.25 billion by 2031"Clinical Trial Investigative Site Network Market" in terms of revenue was estimated to be worth $7.99 billion in 2023 and is poised to reach $14.25 billion by 2031, growing at a CAGR of 7.70% from 2024 to 2031 according to a new report by InsightAce Analytic.
Request for Sample Pages: [https://www.insightaceanalytic.com/request-sample/1486]
Latest Drivers Restraint and Opportunities Market Snapshot:
Key factors influencing the clinical trial investigative site network market are:
* Medical Technology Advancements
* Increasing Interest in Clinical Trials
* Increasing Chronic Diseases
The following are the primary obstacles to the clinical trial investigative site network market 's expansion:
* High cost
* Restrictive Government Standards
* Problems with Attracting and Keeping Patients
Future expansion opportunities for the clinical trial investigative site network market include:
* Investment Research and Development
* Growth in Up-and-coming Industries
* Advancements in Technology
Market Analysis:
The Clinical Trial Investigative Site Network facilitates collaboration between sponsors and CROs to conduct clinical trials. Hospitals, clinics, research centers, and other specialized facilities that fulfill the precise standards for trials may be used as sites. The main drivers of industry growth include an increase in clinical trials conducted worldwide, a rise in the need to lower the expenses related to choosing trial sites, and an increasing need to raise the standard of clinical studies.
List of Prominent Players in the Clinical Trial Investigative Site Network Market:
* ICON Plc
* Meridian Clinical Research
* IQVIA Inc.
* Clinedge
* WCG
* ClinChoice
* Access Clinical Research
* FOMAT Medical Research, Inc.
* SGS
* KV Clinical
* SMO-Pharmina
* Xylem Clinical Research
* Aurum Clinical Research
Clinical Trial Investigative Site Network Market Report Scope:
Report Attribute
Specifications
Market size value in 2023
USD 7.99 billion
Revenue forecast in 2031
USD 14.25 billion
Growth rate CAGR
CAGR of 7.70% from 2024 to 2031
Quantitative units
Representation of revenue in US$ Million, and CAGR from 2024 to 2031
Historic Year
2019 to 2023
Forecast Year
2024-2031
Report coverage
The forecast of revenue, the position of the company, the competitive market structure, growth prospects, and trends
Segments covered
By Therapeutic Area, Phase, End-User
Regional scope
North America; Europe; Asia Pacific; Latin America; Middle East & Africa
Recent Developments:
* In January 2024, ICON plc published a whitepaper titled "Optimising biotech funding." This essay will look at where things stand regarding biotech companies and the strategies they might employ for research and development to attract investment and maximize it.
* In November 2023, IQVIA Holdings Inc. was granted a new term B loan of around $1,500 million (the "New Term Loan B"), which was increased in response to lender demand.
* In October 2023, WCG, the world leader in clinical research solution provision, announced the nomination of three significant persons to the board of directors. Joining WCG, Ken Getz, Bob Hugin, and Dr. Amrit Ray provide extensive expertise that will guide the company's future development and value creation.
Curious about this latest version of the report? @ [https://www.insightaceanalytic.com/enquiry-before-buying/1486]
Clinical Trial Investigative Site Network Market Dynamics:
Market Drivers: Medical Technology Advancements
New treatments and medical gadgets have emerged due to the fast development of medical technology. Investigational site networks are in high demand due to the necessity of conducting clinical studies to assess the efficacy of these novel technologies. Medical professionals and government bodies are beginning to recognize the value of evidence-based medicine. For novel medications to be approved and used, there must be proof from clinical trials, which is why investigative site networks are in high demand.
Challenges: High Cost
Clinical trials are subject to a complicated and ever-changing regulatory environment. Compliance with strict regulatory regulations can be expensive and time-consuming for investigative site networks. Clinical trial patient recruitment and retention are not always smooth sailing. Strict eligibility requirements, patient consent, and trial competitiveness are some of the factors that can impede the efficient operation of investigative site networks.
North America is Expected to Grow with the Highest CAGR During the Forecast Period
The clinical trial investigative site network market is likely to register a significant revenue share and develop at a rapid CAGR in the near future. This is because of its huge patient population, supportive regulatory climate, and long-standing healthcare system. The escalating spending on clinical research by American pharmaceutical companies is largely responsible for this big percentage. The need for the area's clinical trial investigation site network is expected to expand due to the large government financing for clinical trials in the US.
Segmentation of Clinical Trial Investigative Site Network Market-
By Therapeutic Area-
* Oncology
* Cardiology
* CNS
* Pain Management
* Endocrine
* Others
By Phase-
* Phase I
* Phase II
* Phase III
* Phase IV
By End-Use-
* Sponsor
* CRO
By Region-
North America-
* The US
* Canada
* Mexico
Europe-
* Germany
* The UK
* France
* Italy
* Spain
* Rest of Europe
Asia-Pacific-
* China
* Japan
* India
* South Korea
* Southeast Asia
* Rest of Asia Pacific
Latin America-
* Brazil
* Argentina
* Rest of Latin America
Middle East & Africa-
* GCC Countries
* South Africa
* Rest of Middle East and Africa
For More Customization @ [https://www.insightaceanalytic.com/customisation/1486]
Media Contact
Company Name: InsightAce Analytic Pvt. Ltd
Contact Person: Diana D'Souza
Email:Send Email [https://www.abnewswire.com/email_contact_us.php?pr=clinical-trial-investigative-site-network-market-report-explores-reviews-analysis-report-2024]
Country: United States
Website: https://www.insightaceanalytic.com/
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Clinical Trial Investigative Site Network Market Report Explores Reviews Analysis Report 2024 here
News-ID: 3459334 • Views: …
More Releases from ABNewswire

Advencar Announces Groundbreaking New Electric Vehicle Model
Advencar, a prominent player in the electric vehicle (EV) industry, is set to launch its latest model, representing a significant leap forward for Chinese EV cars. As one of the foremost Chinese electric car brands, Advencar continues to drive innovation and excellence in the rapidly expanding EV market in China.
"We are thrilled to introduce our newest model, which exemplifies our commitment to cutting-edge technology and sustainable mobility," said Michael Wang,…

Doctor Roya Redefines Luxury - Beyond Materialism: The Emotional Weight of Thing …
Dr. Roya Jafari-Hassad Advocates her Philosophy - Feeling Your Best is the Ultimate Status Symbol
New York, USA - May 19, 2024 - In today's world, luxury is often associated with expensive brands and the latest trends. A coveted designer bag or a high-end watch might seem like the ultimate status symbol, but Dr. Roya Jafari-Hassad, a leading expert in the State of New York, believes it's time to redefine what…

Revolutionizing On-the-Go Jam Sessions: Anygig Guitar Unveils Innovative Designs
In the bustling world of music, where creativity knows no bounds, musicians are constantly seeking instruments that match their nomadic lifestyle without compromising on quality. Enter Anygig Guitar, a trailblazing brand dedicated to crafting travel-friendly instruments that redefine the boundaries of portable music-making. With a diverse lineup featuring headless guitars [https://www.anygigguitar.com/], full-scale models, and everything in between, Anygig Guitar has emerged as the go-to choice for musicians on the move.
"At…

Schneider Electric appoints Ruben Llanes as CEO of Digital Grid
Schneider Electric, the leader in the digital transformation of energy management and automation, has appointed Ruben Llanes as Chief Executive Officer of Digital Grid. Digital Grid aims to accelerate grid modernization, from grid to prosumer, under the pillars of innovation, adoption and partnerships.
Previously CEO of AutoGrid, Llanes delivered growth, introduced improved processes and methods, and launched new solutions, including turnkey VPPs. His tenure culminated with the sale of AutoGrid to…
More Releases for Clinical
Clinical Laboratory Market in Indonesia, Clinical Laboratory Industry in Indones …
"Increase in healthcare expenditure from the Indonesian government has driven the growth of clinical laboratory market in Indonesia."
Increase in Healthcare Awareness: Largely driven by increase in healthcare spending by aging population (~$ 260 per person by 2050), rising income levels, rising awareness for preventive testing, advanced healthcare diagnostic tests offerings, and central government's healthcare measures.
Developments in Testing and Preference for Evidence based testing: There is also a rising number…
Clinical solutions
Are you spending more time with yellow files than with patients? Healthbridge can change that with our intuitive and easy to use clinical platform that is designed specifically for the medical practitioner at the practice.
smaller2
Easily access patient
information
Cloud-based technology enables you to store rich clinical information that can be easily accessed as and when you need it.
Medical billing software innovation
Become a paperless
practice
Create scripts, sick notes, and clinical notes electronically. Plus, have…
Clinical Research and Clinical Trials Summit
Clinical Research 2019 has been designed in an interdisciplinary manner with a multitude of tracks to choose from every segment and provides you with a unique opportunity to meet up with peers from both industry and academia and establish a scientific network between them. We cordially invite all concerned people to come join us at our event and make it successful by your participation.
This is the premier interdisciplinary forum for…
Paediatric Clinical Trial Conference - When designing a Paediatric clinical tria …
Press Release – 12.02.2018
When designing a Paediatric clinical trial, a paediatric investigation plan (PIP) is a development plan aimed at ensuring that the necessary data are obtained through studies in children, to support the authorisation of a medicine for children. All applications for marketing authorisation for new medicines have to include the results of studies as described in an agreed PIP, unless the medicine is exempt because of a deferral…
Clinical Communication
According to a recent market report published by Persistence Market Research titled “Clinical Communication and Collaboration Market: Global Industry Analysis (2012–2016) and Forecast (2017–2025),” revenue from the global clinical communication and collaboration market was US$ 138.5 Mn in 2012 and US$ 214.8 Mn in 2016, representing a CAGR of 11.6% from 2012 to 2016. This revenue growth is attributed to addition of new features in clinical communication and collaboration solutions.…
E-Clinical Trial Solutions Market To Accelerating Clinical Development Technolog …
The study of the "Global e-Clinical Trial Solutions Market" provides the market size information and market trends along with the factors and parameters impacting it in both short and long term. The study ensures a 360° view, bringing out the complete key insights of the industry.
The Global e-Clinical Trial Solutions Market Research Report Forecast 2017-2021 is a valuable source of insightful data for business strategists. It provides the e-Clinical…