Press release
Future Outlook for the Global Clinical Trials Support Services Market in 2028
As per Triton's report, the global clinical trials support services market attained $20677.01 million in 2021. It is anticipated to register a CAGR of 7.84% by 2028.A recent study by Triton Market Research titled 'Global Clinical Trials Support Services Market' includes Analysis and Forecasts by Service (Administrative Services, Data Management, Patient Recruitment Management, Regulatory Services, Clinical Trial Site Management, Other Services), by Sponsor (Medical Device Companies, Pharmaceutical & Biopharmaceutical Companies, Other Sponsors), by Phase Type (Phase I, Phase II, Phase III, Phase IV), and by Regional Outlook (Middle East and Africa, North America, Asia-Pacific, Europe, and Latin America).
Read the Market Summary Here:
https://www.tritonmarketresearch.com/reports/clinical-trials-support-services-market
Clinical trials are various experiments conducted on humans and animals to examine the outcomes of certain drugs, vaccines, biologics, etc. They are carried out in different phases, such as from stages I to IV, to evaluate their potency and probable side effects.
Request a Free Sample of the Global Clinical Trials Support Services Market Report @ https://www.tritonmarketresearch.com/reports/clinical-trials-support-services-market#request-free-sample
Triton Market Research's report indicates that the global market for clinical trials support services is likely to progress at a CAGR of 7.84% from 2022 to 2028, reaching a revenue worth $34994.75 million by 2028.
Here's a link to our Regional Reports Summary:
https://www.tritonmarketresearch.com/search-report/CLINICAL+TRIALS+SUPPORT+SERVICES+MARKET
The necessity to go over the obstacles in clinical trials-which can be made easier with the help of support services-is probably what will drive the market's growth. The market is facing significant challenges due to the rising cost of clinical trials and associated variables including complexity, duration, and dangers. As a result, many strategies, including data management and clinical trial site management, have been developed to address these issues and are provided by CROs in an effort to improve the effectiveness and value of trial procedures.
The use of information technology is essential in the healthcare sector. For the clinical trial process, major firms provide a variety of services, products, and software, including open-source software. This kind of software is dependable, well-made, and has several advantages, including quicker diagnosis that lead to more successful treatments and shorter paths to cures. It must be used carefully, though.Using open-source software raises the danger of hacking and data breaches if extra security precautions are not taken. Thus, market expansion may be hampered.
The clinical trials support services market is divided into service, sponsor, and phase type. The service segment includes administrative services, data management, patient recruitment management, regulatory services, clinical trial site management, and other services. The sponsor segment is divided into medical device companies, pharmaceutical & biopharmaceutical companies, and other sponsors. And lastly, the phase type segment is branched out into phase I, phase II, phase III, and phase IV.
The Asia-Pacific is expected to be the fastest-growing region in the global market over the considered duration. This market is mainly driven by the surge in the number of trial studies being conducted. From 2008 to 2017, a seven-fold increase in the number of registered clinical trials performed was reported in Asia. The increase in clinical trials may be ascribed to the presence of diverse demographic groupings and the availability of numerous treatment-inexperienced patients in the region. Additionally, the high prevalence rate of certain diseases in some Asian countries makes them the preferred recruitment site for such disease treatment trials.
The established players in the clinical trials support services market include CTI Clinical Trial and Consulting Services Inc, Medpace, Alcura, WuXi AppTec, Syneos Health, Labcorp Drug Development, KCR, Parexel, PSI CRO, Clinipace, Worldwide Clinical Trials, ICON Plc, Charles River Laboratories, Advanced Clinical, and IQVIA.
Purchase this Report @ https://www.tritonmarketresearch.com/reports/clinical-trials-support-services-market#purchase-option
Question & Answer: Clinical Trials Support Services Market
Question 1: Which factor fuels the clinical trials support services market?
Answer: The market expansion is likely to be fueled by the need to overcome the barriers in clinical trials, which can be facilitated with the use of support services. The increasing cost of clinical trials has become a major challenge for the market, along with factors like time, complexity, and risks involved.
Thus, various solutions, such as clinical trial site management, data management, and others, have emerged to overcome these challenges, and are offered by CROs to make trial processes more efficient and valuable.
Question 2: Which factor impedes the clinical trials support services market's growth?
Answer: Information technology plays a vital role in the healthcare industry. Key players are offering different solutions, services, or software for the clinical trial process, such as open-source software. This type of software is reliable and well-designed and offers several benefits, like shorter routes to cures and faster diagnostics, resulting in more effective treatments.
However, it must be used with discretion. If additional security measures are not taken while using open-source software, it increases the risk of hacking and data breaches. This, in turn, can hinder market growth.
Question 3: Which is the fastest-growing region in the clinical trials support services market?
Answer: The Asia-Pacific is expected to be the fastest-growing region in the global market over the considered duration. This market is mainly driven by the surge in the number of trial studies being conducted. From 2008 to 2017, a seven-fold increase in the number of registered clinical trials performed was reported in Asia.
The increase in clinical trials may be ascribed to the presence of diverse demographic groupings and the availability of numerous treatment-inexperienced patients in the region. Additionally, the high prevalence rate of certain diseases in some Asian countries makes them the preferred recruitment site for such disease treatment trials.
Question 4: Which are the established firms in the clinical trials support services market?
Answer: The established players in the clinical trials support services market include CTI Clinical Trial and Consulting Services Inc, Medpace, Alcura, WuXi AppTec, Syneos Health, Labcorp Drug Development, KCR, Parexel, PSI CRO, Clinipace, Worldwide Clinical Trials, ICON Plc, Charles River Laboratories, Advanced Clinical, and IQVIA.
Related Report:
Global Clinical Trial Management Market-
https://www.tritonmarketresearch.com/reports/clinical-trial-management-market
The global clinical trial management market is expected to generate a revenue of approximately $1810.09 million by 2028, growing at a CAGR of 10.92% during the forecast period 2021-2028.
Market drivers such as the rise in chronic diseases, increased medical research & development, and the benefits of CTM software fuel the growth of the studied market. Research in the healthcare industry is rising owing to the growth in the patient base worldwide and the need for better quality healthcare.
The governments of different nations plan to enhance their investments in healthcare research to meet the quality standards, develop newer & better technologies, and cater to the needs of patients. For instance, the Economics of Industrial Research & Innovation (IRI) shows that R&D intensity in the research-based pharmaceutical industry in Europe amounts to 13.3%.
Such growth in research activities has consequently increased the number of clinical trials conducted. This serves as a growth factor for the global clinical trial management market.
Triton Market Research
196, wards wharf approach
London E16 2EQ
Phone: +44 7441 911839
Email: sales@tritonmarketresearch.com
Website: https://www.tritonmarketresearch.com/
Triton is a leading market research company providing clients with the best online market research data reports.Our offerings include syndicated market insights, customized research reports, and cost-effective consulting services for constructive decision-making.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Future Outlook for the Global Clinical Trials Support Services Market in 2028 here
News-ID: 3373821 • Views: …
More Releases from Triton Market Research
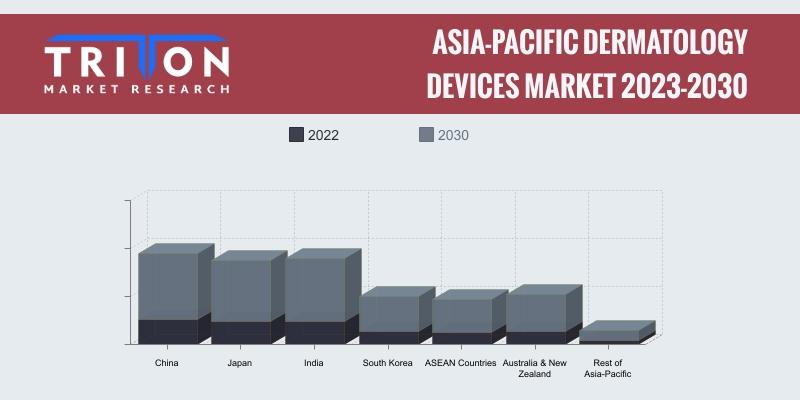
Asia-Pacific Dermatology Devices Market Forecast 2023-2030: Trends and Growth An …
The Asia-Pacific Dermatology Devices Market is experiencing significant growth, driven by increasing awareness and prevalence of skin disorders. Countries within this market include China, Japan, South Korea, India, Australia & New Zealand, ASEAN countries, and the Rest of Asia-Pacific. According to a research report by Triton, the market is expected to grow at a compound annual growth rate (CAGR) of 13.36% from 2023 to 2030.
Read the Market Summary Here: https://www.tritonmarketresearch.com/reports/asia-pacific-dermatology-devices-market#report-overview?utm_source=PaidPRNew&utm_medium=OpenPR&utm_campaign=TritonPR…

Future Insights: Middle East and Africa Dermatology Devices Market Forecast 2023 …
The MIDDLE EAST AND AFRICA DERMATOLOGY DEVICES MARKET is poised for substantial growth, driven by the rising prevalence of skin disorders and increasing demand for advanced dermatological treatments. The market includes countries such as the United Arab Emirates, Saudi Arabia, Turkey, South Africa, and the Rest of the Middle East & Africa. According to Triton's research report, the dermatology devices market in this region is expected to advance at a…

Expanding Scope of the Global Membranes Market in 2024-2032
According to Triton's research report, the global membranes market generated $XX million in 2023 and is expected to generate a CAGR of 6.74% in revenue over the forecasting years 2024-2032.
A recent study by Triton Market Research titled Global Membranes Market includes the Global Analysis and Forecasts by Material (Polymeric, Ceramic, Other Materials), Technology(Reverse Osmosis (RO), Nanofiltration (NF), Ultrafiltration (UF), Microfiltration (MF), Other Technologies), End-User (Utilities, Commercial, Industrial, Residential, Other End-Users),…
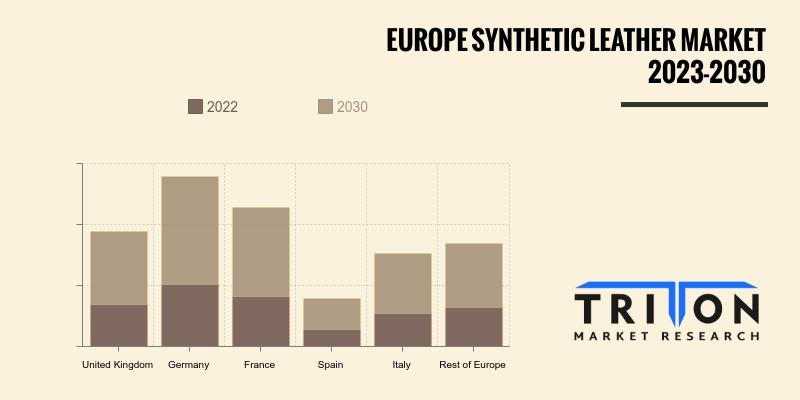
Europe Synthetic Leather Market Size & Forecast to 2030
Triton Market Research states that the Europe Synthetic Leather Market is poised for significant expansion, with an anticipated compound annual growth rate (CAGR) of 7.23% in revenue from 2023 to 2030.
Read the Market Summary Here: https://www.tritonmarketresearch.com/reports/europe-synthetic-leather-market#report-overview?utm_source=PaidPRNew&utm_medium=OpenPR&utm_campaign=TritonPR
This growth is driven by increasing consumer preference for cruelty-free and sustainable alternatives to genuine leather, as well as advancements in synthetic leather technology.
Countries leading this market include Spain, Italy, France, Germany, the…
More Releases for Clinical
Clinical Laboratory Market in Indonesia, Clinical Laboratory Industry in Indones …
"Increase in healthcare expenditure from the Indonesian government has driven the growth of clinical laboratory market in Indonesia."
Increase in Healthcare Awareness: Largely driven by increase in healthcare spending by aging population (~$ 260 per person by 2050), rising income levels, rising awareness for preventive testing, advanced healthcare diagnostic tests offerings, and central government's healthcare measures.
Developments in Testing and Preference for Evidence based testing: There is also a rising number…
Clinical solutions
Are you spending more time with yellow files than with patients? Healthbridge can change that with our intuitive and easy to use clinical platform that is designed specifically for the medical practitioner at the practice.
smaller2
Easily access patient
information
Cloud-based technology enables you to store rich clinical information that can be easily accessed as and when you need it.
Medical billing software innovation
Become a paperless
practice
Create scripts, sick notes, and clinical notes electronically. Plus, have…
Clinical Research and Clinical Trials Summit
Clinical Research 2019 has been designed in an interdisciplinary manner with a multitude of tracks to choose from every segment and provides you with a unique opportunity to meet up with peers from both industry and academia and establish a scientific network between them. We cordially invite all concerned people to come join us at our event and make it successful by your participation.
This is the premier interdisciplinary forum for…
Paediatric Clinical Trial Conference - When designing a Paediatric clinical tria …
Press Release – 12.02.2018
When designing a Paediatric clinical trial, a paediatric investigation plan (PIP) is a development plan aimed at ensuring that the necessary data are obtained through studies in children, to support the authorisation of a medicine for children. All applications for marketing authorisation for new medicines have to include the results of studies as described in an agreed PIP, unless the medicine is exempt because of a deferral…
Clinical Communication
According to a recent market report published by Persistence Market Research titled “Clinical Communication and Collaboration Market: Global Industry Analysis (2012–2016) and Forecast (2017–2025),” revenue from the global clinical communication and collaboration market was US$ 138.5 Mn in 2012 and US$ 214.8 Mn in 2016, representing a CAGR of 11.6% from 2012 to 2016. This revenue growth is attributed to addition of new features in clinical communication and collaboration solutions.…
E-Clinical Trial Solutions Market To Accelerating Clinical Development Technolog …
The study of the "Global e-Clinical Trial Solutions Market" provides the market size information and market trends along with the factors and parameters impacting it in both short and long term. The study ensures a 360° view, bringing out the complete key insights of the industry.
The Global e-Clinical Trial Solutions Market Research Report Forecast 2017-2021 is a valuable source of insightful data for business strategists. It provides the e-Clinical…
