Press release
Familial Chylomicronemia Syndrome Market 2032: Epidemiology Insights, Pipeline Updates, Regulatory Milestones by DelveInsight | Arrowhead Pharma, Visirna Therapeutics HK Ltd, Ionis Pharma, Novartis
The Familial Chylomicronemia Syndrome Market size was valued approximately USD 15 million in 2022 and the report offers an in-depth understanding of the Familial Chylomicronemia Syndrome, historical and forecasted epidemiology as well as the Familial Chylomicronemia Syndrome market trends in the 7MM.DelveInsight's "Familial Chylomicronemia Syndrome Market Insights, Epidemiology, and Market Forecast-2032 report offers an in-depth understanding of the Familial Chylomicronemia Syndrome, historical and forecasted epidemiology as well as the Familial Chylomicronemia Syndrome market trends in the United States, EU4 (Germany, Spain, Italy, France) the United Kingdom and Japan.
To Know in detail about the Familial Chylomicronemia Syndrome market outlook, drug uptake, treatment scenario and epidemiology trends, Click here; Familial Chylomicronemia Syndrome Market Forecast [https://www.delveinsight.com/sample-request/familial-chylomicronemia-syndrome-market?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=gpr]
Some of the key facts of the Familial Chylomicronemia Syndrome Market Report:
*
The Familial Chylomicronemia Syndrome market size was valued approximately USD 15 million in 2022 and is anticipated to grow with a significant CAGR during the study period (2019-2032)
*
In May 2023, Arrowhead Pharmaceuticals has finished enrolling participants for its Phase III PALISADE trial investigating ARO-APOC3 for treating familial chylomicronemia syndrome (FCS), an extremely rare genetic disorder typically caused by various single-gene mutations. The international AROAPOC3-3001 PALISADE trial will evaluate the effectiveness and safety of the investigational RNAi therapeutic ARO-APOC3 in adult patients with FCS.
*
In the 7MM, there are now 2,989 diagnosed prevalent cases of FCS, with an expected rise to 3,032 cases by 2032. In terms of diagnosed prevalence, the US was followed by Japan
*
Nearly 22.9% of the 7MM's diagnosed prevalent FCS cases were located in the US in 2021, and by 2032, that percentage is projected to rise even further
*
Nemeth et al. (2022), who examined the medical data of 1,342,124 patients, calculated that the frequency of FCS in the region of Central Europe was 19.4 per million. It's possible that the variation in disease prevalence was caused by the study's use of a population that was treated or examined in a hospital
*
In 2021, the United States had about 1340 prevalent cases, a number anticipated to rise by 2032.
*
Key Familial Chylomicronemia Syndrome Companies: Arrowhead Pharma, Visirna Therapeutics HK Limited, Ionis Pharmac, Novartis, Akcea Therapeutics, and others
*
Key Familial Chylomicronemia Syndrome Therapies: ARO-APOC3, VSA001 injection, Olezarsen, LCQ908, AKCEA-ANGPTL3-LRx, Volanesorsen, and others
*
The Familial Chylomicronemia Syndrome market is expected to surge due to the disease's increasing prevalence and awareness during the forecast period. Furthermore, launching various multiple-stage Familial Chylomicronemia Syndrome pipeline products will significantly revolutionize the Familial Chylomicronemia Syndrome market dynamics.
Familial Chylomicronemia Syndrome Overview
An extremely rare hereditary condition known as familial chylomicronemia syndrome (FCS) inhibits the body from metabolising dietary fats, or triglycerides, that are eaten. Large structures in the blood known as chylomicrons assist transport these triglycerides to various locations throughout the body where they are required for energy production and fat storing.
Get a Free sample for the Familial Chylomicronemia Syndrome Market Report:
https://www.delveinsight.com/report-store/familial-chylomicronemia-syndrome-market [https://www.delveinsight.com/report-store/familial-chylomicronemia-syndrome-market?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=gpr]
Familial Chylomicronemia Syndrome Epidemiology
The epidemiology section provides insights into the historical, current, and forecasted epidemiology trends in the seven major countries (7MM) from 2019 to 2032. It helps to recognize the causes of current and forecasted trends by exploring numerous studies and views of key opinion leaders. The epidemiology section also provides a detailed analysis of the diagnosed patient pool and future trends.
Familial Chylomicronemia Syndrome Epidemiology Segmentation:
The Familial Chylomicronemia Syndrome market report proffers epidemiological analysis for the study period 2019-2032 in the 7MM segmented into:
*
Total Prevalence of Familial Chylomicronemia Syndrome
*
Prevalent Cases of Familial Chylomicronemia Syndrome by severity
*
Gender-specific Prevalence of Familial Chylomicronemia Syndrome
*
Diagnosed Cases of Episodic and Chronic Familial Chylomicronemia Syndrome
Download the report to understand which factors are driving Familial Chylomicronemia Syndrome epidemiology trends @ Familial Chylomicronemia Syndrome Epidemiology Forecas [https://www.delveinsight.com/sample-request/familial-chylomicronemia-syndrome-market?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=gpr]t
Familial Chylomicronemia Syndrome Drugs Uptake and Pipeline Development Activities
The drugs uptake section focuses on the rate of uptake of the potential drugs recently launched in the Familial Chylomicronemia Syndrome market or expected to get launched during the study period. The analysis covers Familial Chylomicronemia Syndrome market uptake by drugs, patient uptake by therapies, and sales of each drug.
Moreover, the therapeutics assessment section helps understand the drugs with the most rapid uptake and the reasons behind the maximal use of the drugs. Additionally, it compares the drugs based on market share.
The report also covers the Familial Chylomicronemia Syndrome Pipeline Development Activities. It provides valuable insights about different therapeutic candidates in various stages and the key companies involved in developing targeted therapeutics. It also analyzes recent developments such as collaborations, acquisitions, mergers, licensing patent details, and other information for emerging therapies.
Familial Chylomicronemia Syndrome Therapies and Key Companies
*
ARO-APOC3: Arrowhead Pharma
*
VSA001 injection: Visirna Therapeutics HK Limited
*
Olezarsen: Ionis Pharmac
*
LCQ908: Novartis
*
AKCEA-ANGPTL3-LRx: Akcea Therapeutics
*
Volanesorsen: Akcea Therapeutics
Discover more about therapies set to grab major Familial Chylomicronemia Syndrome market share @ Familial Chylomicronemia Syndrome Treatment Market [https://www.delveinsight.com/sample-request/familial-chylomicronemia-syndrome-market?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=gpr]
Scope of the Familial Chylomicronemia Syndrome Market Report
*
Study Period: 2019-2032
*
Coverage: 7MM [The United States, EU5 (Germany, France, Italy, Spain, and the United Kingdom), and Japan]
*
Key Familial Chylomicronemia Syndrome Companies: Arrowhead Pharma, Visirna Therapeutics HK Limited, Ionis Pharmac, Novartis, Akcea Therapeutics, and others
*
Key Familial Chylomicronemia Syndrome Therapies: ARO-APOC3, VSA001 injection, Olezarsen, LCQ908, AKCEA-ANGPTL3-LRx, Volanesorsen, and others
*
Familial Chylomicronemia Syndrome Therapeutic Assessment: Familial Chylomicronemia Syndrome current marketed and Familial Chylomicronemia Syndrome emerging therapies
*
Familial Chylomicronemia Syndrome Market Dynamics: Familial Chylomicronemia Syndrome market drivers and Familial Chylomicronemia Syndrome market barriers
*
Competitive Intelligence Analysis: SWOT analysis, PESTLE analysis, Porter's five forces, BCG Matrix, Market entry strategies
*
Familial Chylomicronemia Syndrome Unmet Needs, KOL's views, Analyst's views, Familial Chylomicronemia Syndrome Market Access and Reimbursement
To know more about Familial Chylomicronemia Syndrome companies working in the treatment market, visit @ Familial Chylomicronemia Syndrome Clinical Trials and Therapeutic Assessment [https://www.delveinsight.com/sample-request/familial-chylomicronemia-syndrome-market?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=gpr]
Table of Contents
1. Familial Chylomicronemia Syndrome Market Report Introduction
2. Executive Summary for Familial Chylomicronemia Syndrome
3. SWOT analysis of Familial Chylomicronemia Syndrome
4. Familial Chylomicronemia Syndrome Patient Share (%) Overview at a Glance
5. Familial Chylomicronemia Syndrome Market Overview at a Glance
6. Familial Chylomicronemia Syndrome Disease Background and Overview
7. Familial Chylomicronemia Syndrome Epidemiology and Patient Population
8. Country-Specific Patient Population of Familial Chylomicronemia Syndrome
9. Familial Chylomicronemia Syndrome Current Treatment and Medical Practices
10. Familial Chylomicronemia Syndrome Unmet Needs
11. Familial Chylomicronemia Syndrome Emerging Therapies
12. Familial Chylomicronemia Syndrome Market Outlook
13. Country-Wise Familial Chylomicronemia Syndrome Market Analysis (2019-2032)
14. Familial Chylomicronemia Syndrome Market Access and Reimbursement of Therapies
15. Familial Chylomicronemia Syndrome Market Drivers
16. Familial Chylomicronemia Syndrome Market Barriers
17. Familial Chylomicronemia Syndrome Appendix
18. Familial Chylomicronemia Syndrome Report Methodology
19. DelveInsight Capabilities
20. Disclaimer
21. About DelveInsight
About DelveInsight
DelveInsight is a leading Healthcare Business Consultant, and Market Research firm focused exclusively on life sciences. It supports Pharma companies by providing comprehensive end-to-end solutions to improve their performance.
It also offers Healthcare Consulting Services, which benefits in market analysis to accelerate the business growth and overcome challenges with a practical approach.
Media Contact
Company Name: DelveInsight
Contact Person: Gaurav Bora
Email:Send Email [https://www.abnewswire.com/email_contact_us.php?pr=familial-chylomicronemia-syndrome-market-2032-epidemiology-insights-pipeline-updates-regulatory-milestones-by-delveinsight-arrowhead-pharma-visirna-therapeutics-hk-ltd-ionis-pharma-novartis]
Phone: +14699457679
Address:304 S. Jones Blvd #2432
City: Las Vegas
State: NV
Country: United States
Website: https://www.delveinsight.com/
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Familial Chylomicronemia Syndrome Market 2032: Epidemiology Insights, Pipeline Updates, Regulatory Milestones by DelveInsight | Arrowhead Pharma, Visirna Therapeutics HK Ltd, Ionis Pharma, Novartis here
News-ID: 3491606 • Views: …
More Releases from ABNewswire

PGIM and SIPioneer Announce Strategic Collaboration to Develop Super AIPGIM Quan …
PGIM, a global leader in investment management, is proud to announce a strategic partnership with Smart Investment Pioneer (SIPioneer [https://website.tourpioneer.com/]), a trailblazer in the financial services industry. Together, they are embarking on a groundbreaking project to develop the advanced Super AIPGIM quantitative trading system. This collaboration aims to revolutionize investment advisory services by combining the strengths of both companies to provide superior investment advice and enhance the precision and reliability…
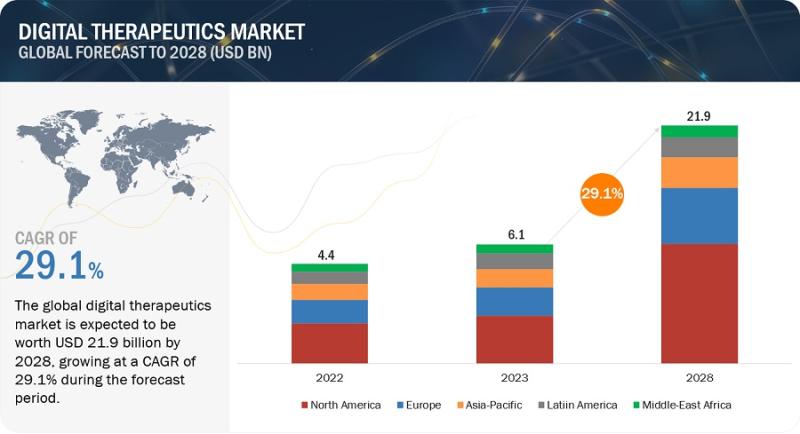
Digital Therapeutics Market Application, Growth, Trends, Size, Opportunities, To …
Digital Therapeutics (DTx) Market in terms of revenue was estimated to be worth $6.1 billion in 2023 and is poised to reach $21.9 billion by 2028, growing at a CAGR of 29.1% from 2023 to 2028 according to a new report by MarketsandMarkets Trademark .
The Rise of Digital Therapeutics: Transforming Healthcare
The healthcare industry is witnessing a revolutionary shift with the advent of digital therapeutics (DTx) [https://www.prnewswire.com/news-releases/digital-therapeutics-dtx-market-worth-21-9-billion--marketsandmarkets-302041683.html]. Unlike traditional treatments, digital…

Industry Update: Dr. Reddy's Growth Trajectory: Breaking Down the Pharma Giant's …
Dr. Reddy's Laboratories Ltd, a global pharmaceutical leader, has reported its highest ever revenues and profits for the fiscal year 2024. With revenues surpassing $3.3 billion and EBITDA at the highest levels, the company has achieved a milestone in its 40-year history of serving patients and other stakeholders worldwide.
Strong Financial Performance
Dr. Reddy's consolidated revenues for the fiscal year 2024 reached a historic high of $3.3 billion, marking a significant 14%…

Revolutionizing Trucking: How Portal Transport Solutions is Paving the Way with …
In an industry as dynamic and essential as trucking, efficiency, and reliability are the cornerstones of success. Portal Transport Solutions (PTS) stands out as a beacon of innovation, providing comprehensive solutions through its Fleet Builder Program and expert driver staffing services. With glowing reviews from satisfied clients, PTS is setting new standards in the trucking industry, ensuring businesses not only survive but thrive.
The Fleet Builder Program: A Turnkey Solution
The PTS…
More Releases for Familial
Familial Adenomatous Polyposis (FAP) Therapeutics- Pipeline Analysis 2018
Familial adenomatous polyposis (FAP) is a genetic disorder, characterized by the development of adenomatous colon polyps in the intestinal tract, resulting in colon cancer. The disease is diagnosed when more than 100 adenomatous colon polyps are developed in a person.
Download the sample report at: https://www.pharmaproff.com/request-sample/1025
A person with FAP is at a high risk of developing cancer of small intestines or stomach. This disease is categorized into three types including attenuated…
Familial Amyloid Cardiomyopathy Treatment Market Opportunity Analysis, 2018–20 …
Aggregation and deposition of mutant and wild-type transthyretin protein (TTR) in heart results in familial amyloid cardiomyopathy, which typically occur after age of 60. Familial amyloid cardiomyopathy is also known as hereditary cardiac transthyretin amyloidosis or hereditary amyloid cardiomyopathy. The protein transthyretin amyloid fibrils infiltrate the myocardium that leads to diastolic dysfunction from restrictive cardiomyopathy, which eventually result to heart failure. There are several mutation in TTR which are associated…
Heterozygous familial hypercholesterolemia (heFH) - Pipeline Review, H2 2017
Market Research Hub's latest Pharmaceutical and Healthcare disease pipeline guide “Heterozygous familial hypercholesterolemia (heFH) - Pipeline Review”, H2 2017, provides an overview of the Heterozygous familial hypercholesterolemia (heFH) (Metabolic Disorders) pipeline landscape.
Heterozygous familial hypercholesterolemia (HeFH) is a genetic disorder caused due to a mutation (alteration) of FH from one (affected) parent. Symptoms include xanthesmas, corneal arcus, aortic rupture and peripheral vascular disease. Risk factors include age, sex, smoking and hypertension,…
Familial Adenomatous Polyposis - Pipeline Review, H2 2017
ReportsWorldwide has announced the addition of a new report title Familial Adenomatous Polyposis - Pipeline Review, H2 2017 to its growing collection of premium market research reports.
Summary
Global Markets Direct's latest Pharmaceutical and Healthcare disease pipeline guide Familial Adenomatous Polyposis - Pipeline Review, H2 2017, provides an overview of the Familial Adenomatous Polyposis (Genetic Disorders) pipeline landscape.
Familial adenomatous polyposis (FAP) or classic FAP is a genetic condition that causes extra…
Familial Chylomicronemia Syndrome Treatment Market
Familial Chylomicronemia Syndrome is a genetic condition characterized by an inability of the body to digest fats mainly triglyceride. In Familial Chylomicronemia Syndrome, the lipoprotein lipase (LPS) is not functional, which is the enzyme that breaks down chylomicrons in the blood. The most distinctive sign of Familial Chylomicronemia Syndrome is the appearance of fatty blood on the skin, mainly owing to high level of fat in the body. People with…
Familial Adenomatous Polyposis - Pipeline Review, H1 2017
ReportsWorldwide has announced the addition of a new report title Familial Adenomatous Polyposis - Pipeline Review, H1 2017 to its growing collection of premium market research reports.
Global Markets Direct's latest Pharmaceutical and Healthcare disease pipeline guide Familial Adenomatous Polyposis - Pipeline Review, H1 2017, provides an overview of the Familial Adenomatous Polyposis (Genetic Disorders) pipeline landscape.
Familial adenomatous polyposis (FAP) is also known as familial polyposis coli, adenomatous polyposis coli, or…
